3
Ghosts in the Machine: Publication Planning 101
Three Puzzles
I was looking at the CV of a distinguished professor of medicine, and saw that he had authored (generally co-authored) approximately 800 articles in peer-reviewed journals, an average of nearly thirty per year over his career. His publication rate has accelerated, and he has been authoring forty articles per year in the past decade. How can a scientist publish forty articles in a year? Year after year? In the fields in which I work, five peer-reviewed articles in a year is respectable.
I was looking at the articles published on a blockbuster drug (i.e. sales over $1 billion per year). The PubMed database contained over 700 articles in the ‘core clinical journals’ that showed that drug’s generic name as a keyword. There were over 3200 articles on the drug in medical journals as a whole. Other blockbuster drugs have very similar profiles. Why do these drugs merit such attention?
I was part of a research project that systematically compared industry-sponsored published studies with apparently independent ones. We did a statistical summary – a ‘meta-analysis’ – of previous efforts to compare sponsored and independent medical research; the comparison involved nearly four thousand different medical studies. The industry-sponsored publications were significantly and strikingly more likely to arrive at industry-friendly results than were the apparently independent publications.1 How could mere sponsorship lead researchers to come to results that favour their sponsors? Can research funding really have such strong effects?
This triple mystery has a single solution. The pharmaceutical industry produces an abundance of targeted knowledge, flooding its important markets. To gain the largest scientific impact and market value from research, drug company articles are often written under the names of independent medical researchers. Pharma company statisticians, reviewers from a diverse array of company departments, medical writers, and publication planners are only rarely acknowledged in journal publications, and company scientists only sometimes acknowledged. The public knowledge that results from this ghost-managed research and publication is a marketing tool, providing bases for continuing medical education, buttressing sales pitches, and contributing to medical common sense and further research. In the world of pharma, knowledge is a resource to be accumulated, shaped, and deployed to best effect.
The Ghosts Behind Pharma’s Medical Publications
When I first became interested in pharmaceutical research and marketing, a number of people in medicine were talking about ghostwriting. It was common, it seemed, for medical journal articles to be written by professional writers working for pharma companies. Those writers’ names did not appear on the articles themselves, which were instead ‘authored’ mostly by medical researchers. Clearly, this was a scandal.
It is an understatement to say that university researchers can be competitive. Since published articles are one of the main currencies of prestige for academics, many medical researchers first see the scandal of ghostwriting in terms of injustice, the injustice of guest authors taking credit for work that they didn’t do. It is only on further reflection that they extend the blame to include ghostwriters and the pharmaceutical industry.
Surprisingly, the people talking about medical ghostwriting are rarely curious about the larger structures in which ghosts appear. It is as if ghostwriting is something that happens frequently, but on a purely one-off basis. This can’t be right, especially since pharma companies are large organizations and so must have structures to handle ghostwriting. When I became interested in pharma’s publications, I immediately became curious about background questions, such as: Who hires the writers? How do they know what to write? How is the work planned and executed? How many articles are ghostwritten? In trying to answer these questions, I quickly stumbled across ‘publication planning’, and started to study who publication planners are and what they do.2 When we focus on publication planning, the guest authors and ghostwriters start to seem like a bit of a distraction from more important ghosts.
Publication of drug company research in medical science journals, and its presentation at conferences and meetings, is governed by ‘publication plans’. These plans extract scientific and commercial value out of data and analyses, sometimes by designing studies with that value in mind, and always by carefully constructing articles that establish consistent profiles for drugs. As we’ve seen, most sponsored clinical trial research is handled by contract research organizations (CROs). The data these CROs produce is typically analysed by pharma company statisticians, and then articles are written by hired medical writers. Much of this process is guided and shepherded by publication planners and planning teams.
The manuscripts are ‘authored’ by academic researchers, whose contribution may range from having been on a company advisory board related to the study, to having supplied some of the patients for a clinical trial, to editing the manuscript, to simply signing off on the final draft. The publication planners then submit the manuscripts to medical journals, where they are generally well received and are published. While these published articles contribute to accepted scientific opinions, the circumstances of their production are largely invisible. When they are useful, they form the basis for presentations by hired doctors and researchers. Marketing departments of the companies involved may buy thousands of reprints, which sales representatives can give to practising doctors.
It is worth quoting at length one publication plan’s description of planning itself:
Strategic publication planning provides the tactical recommendations necessary to develop a scientific platform within the biomedical literature to support the market positioning of an established product or the launch of a new product. The process of publication planning includes:
- An analysis of the characteristics of the market into which the product will be launched
- An analysis of competitive issues
- The expected product profile
- Identification of issues relevant to the disease state or primary indication for the product
- Development of a series of key communication messages addressing the major issues
- The availability of clinical and preclinical data to support the key communication messages
- Recognition of appropriate target audiences for each of the recommended publication tactics
- Recommendations for publication vehicles (e.g., journals, meetings, congresses, etc.) for each publication activity.3
This very direct description encapsulates much of the rest of this chapter, hitting on all of the major goals and aims of publication planning. In particular, it boldly states that the point of the activity is to position medical science to help market drugs. The science becomes part of the marketing efforts: publication planning creates a ‘scientific platform’ to ‘support market positioning’ of a product. If a drug is a molecule surrounded by information, publication planning helps to create and position that information.
How much of the literature is ghost-managed? From the limited number of cases where we have hard data, it appears that roughly 40% of medical journal articles mentioning in-patent drugs are parts of individual publication plans on the drugs.4 A legal action gave psychiatrist David Healy access to a document listing eighty-five articles on the drug sertraline (Zoloft or Lustral), many of them written by medical writers and then authored by academics, all of them handled for Pfizer by a public relations firm, Current Medical Directions.5 Lawsuits about rofecoxib (Vioxx) led to a systematic study identifying ninety-six published articles (twenty-four on clinical trials and seventy-two review articles) on which Merck had worked prior to their publication, and which were later published mostly under the names of academic first authors. The company Scientific Therapeutics Information wrote a number of articles for Merck, and one document lists eight review articles for which they had intended authors and journals, and estimated delivery dates of first or second drafts. Interestingly, ghost-managed review articles were likely to be single-authored by academics who were especially likely not to declare any support for the work.6 Forty percent is a very substantial amount, certainly allowing a company to attract interest in a drug and shape the perception of it, under the names of apparently independent authors.
Various facts make it reasonable to believe that thousands of articles per year are ghost-managed. First, pharma companies sponsor some 70% of all clinical trials, and 70-75% of these are run by CROs that have no interest in publishing the results under their own names – they produce data that is wholly owned by their sponsors. As a result, pharma companies have complete control over an enormous trove of clinical trial data. Second, more than fifty agencies advertise publication planning on the internet. Some boast of having hundreds of employees and handling many hundreds of manuscripts per year. Planners handle dozens of manuscripts per year, and one told me that she was in charge of a campaign involving more than a hundred manuscripts and conference presentations. The industry is large enough that there are two international associations of publication planners that run meetings and seminars. One of these associations, the International Society of Medical Planning Professionals (ISMPP), has over 1000 members. Both ISMPP and its competing association, The International Publication Planning Association (TIPPA) hold annual conferences, and the latter hosts regional conferences. This is a major activity.
Publication Planning 101/201: An Insider View of the Field
To learn more about publication planning, I wanted to hear what planners themselves had to say. My first step was to join ISMPP and register for a workshop, ‘Publication Planning 101/201’, intended for people new to the profession. As a new member of ISMPP, the workshop seemed perfect for me. Immediately following that, I also attended the 2007 annual meetings of ISMPP; over the next decade, I and/or some research associates attended two meetings of TIPPA, and then a European meeting of ISMPP in 2017. The rest of this chapter is an account that draws mostly from presentations at these five events, but also from written sources, to show publication planners’ roles and the structure of pharma’s attempts to shape medical science.
Both ISMPP and TIPPA hold annual conferences, and there are overlaps in their programmes and lists of speakers. ISMPP runs broader educational and accreditation activities, and creates guidelines for ethical and best practices. It is a larger organization than TIPPA, though the latter also holds regional meetings. Almost all attendees of these meetings are publication planners, some working for independent agencies and some directly for pharmaceutical companies; ISMPP is the more agency-dominated of the two. The non-planners are mostly invited speakers, including journal editors, ethicists, and consultants to the industry. Slightly more women than men attend, and at one meeting I estimated the average age of participants at approximately 40 or a little higher; this is a new field, and has few senior figures. Attire is roughly what you might expect in a group of medical writers and scientists working for industry: a range from business suits to business casual, but mostly of the ordinary and slightly rumpled variety. That said, some of the attendees are more ‘corporate’ types – at one small meeting a participant noted the arrival of a contingent from Pfizer, a group of young women who moved as a pack and looked, with their pencil skirt suits and stiletto heels, as though they had walked in directly from Wall Street. Four of the five events reported on in this chapter were in the US, and the fifth in the UK, billed as a European meeting. The US appears to dominate the publication planning world, but the UK is an important second centre.
Publication Planning 101/201 was supposed to provide ‘an interactive and instructive introduction to the world of strategic publication planning’, for those either new to it, working as support to planning, or working in connected areas. Most of the thirty women and thirteen men taking the course were new publication planners, though there were also medical writers, publishing company employees, and more experienced publication planners. Day-long seminars were held simultaneously in adjacent rooms: ‘Publication Planning 301, Developing a Strategic Publication Plan’; ‘The Life of a Manuscript: From Initial Concept to Publication (and Beyond)’; and ‘Statistics for the Non-Statistician, and Publishing Pharmacoeconomics and Outcomes Research’.
The programme for Publication Planning 101/201 began with a history of the field, given by Mr Phillips, a senior member of the field and the CEO of a medium-sized agency. Somewhat artificially, Phillips pointed to 1984 as the origin of publication planning, when three employees of Pfizer realized that the company had extensive data on the drug amlodipine (Norvasc), and wondered where they should publish it. To do this rationally, they had to gather information about all of the trials to which Pfizer had access, harvest information from other publications, sort it all, and decide how to publish it in credible journals for non-overlapping global audiences. The company had to improve internal communication to achieve this. Even by 1988, publication planning was not well established within Pfizer, as demonstrated by an internal memo Phillips quoted: ‘Please … return details of any new trials, new plans for publication of existing trials, or missing details.’ He and some members of the 101/201 audience chuckled, because this sounds quaint today. Close tracking of all trials from their conception onward and top-down guidance of their publication means that ‘[t]oday, if you go to a meeting, you know pretty much what is going to be presented’.
The bare publication plan is a dynamic document that ‘outlines the recommended medical communications and their timings’. However, the activity of publication planning includes the work to implement the plan, to produce the deliverables. Publication planning can and should start even before the research does, contributing to research design, mapping out key messages, charting out articles for different audiences and journals, and finding potential authors for those articles. The focus is communication, and the research is created with this in view. Once the research is available, publication planners hire writers for those articles, deal with potential authors and various interests within the pharma companies, and shepherd the articles through journals’ submission and revision procedures. Publication planning is typically done by heterogeneous teams, and increasingly those teams include one or more professional planners who understand the process of turning data into articles and presentations and guide it. Most of these planners work for dedicated agencies, though pharma companies employ a substantial number directly.
New publication planners are told to pay attention to marketing. Publication planning is a key part of the process of surrounding a molecule with information. In the 101/201 course, Dr Parker explained that a publication plan begins with a SWOT (Strengths, Weaknesses, Opportunities, and Threats) analysis, which ‘paints a complete picture of the market situation for a new product’. In case it isn’t obvious, a SWOT analysis for scientific publications only makes sense if those publications are supposed to serve marketing goals. Shortly afterwards, Dr Price said that publication plans should identify ‘target audiences’, should lay out key ‘scientific & clinical communication points’, should do ‘competitor publication & gap analyses’, and need to outline ‘top-line tactics’ and ‘critical timing’. Clearly, these analyses are parts of the apparatus of interest-driven persuasion, not the disinterested diffusion of results. Similarly, after an exercise in the 101/201 seminar, Parker asked, ‘How are we going to create publications that have the right message, and a memorable message, for prescribers?’ At a later meeting, speaking about his company’s innovative model for evaluating the effects of publications, planner Mr Powers concluded: ‘If you really want to make an impact and leave a footprint with your communication plans, you need to engage your scientific communication plan with activities that engage emotional and social intelligence.’ Former publication planner Alastair Matheson describes the messages as ‘narratives’ that establish consistent profiles for drugs.7
In the opening speech at one publication planning conference, the speaker took it as one of her tasks to cheer on the profession. Holding an imaginary document in her hand and waving it around in the air, she chided an imaginary colleague: ‘What is this? They’re promoting the competitor! Well, you left it to the investigators.’ Another planner agreed shortly afterwards, saying: ‘The approach of an industry-authored first draft is a good one.’
As we’ve seen, there have been substantial changes in the structure of research in the industry since the 1980s, as industry funding moved from supporting academic research to purchasing research from CROs. The simultaneous rise of the publication planning and CRO industries is almost certainly not coincidental, because CROs, unlike academic researchers, make no claims on the data. If scientific data are to be systematically used for marketing, then pharma companies need to have as much control over it as possible. CROs may even do publication planning, which allows them to fully guide research from inception to communication. For example, the website of the CRO Quintiles (which has since merged with IMS Health to form IQVIA) notes that
Effective communications require scientific and commercial specialists who can craft and convey messages backed by evidence and an acute awareness of market and regulatory environments.
And the CRO is in a good position to provide that effective communication:
As the world’s largest provider of biopharmaceutical services, Quintiles offers capabilities that surpass the typical healthcare communications agency. Our singular objective is to increase your probability of success by connecting deep insights with superior delivery for better outcomes.8
A Sample Manuscript (I)
Before turning to more publication planners’ descriptions of what they do, I want to follow a single manuscript that made its way through the publication planning process. The case comes from legal documents that were made public.9
The drug company Wyeth has faced thousands of lawsuits to do with over-promotion of hormone replacement therapy (HRT); it has lost most of the first handful of cases to be decided. Because of these suits, a number of documents have become available for public scrutiny.10 We know, for example, that in the late 1990s and early 2000s, Wyeth turned to the medical education and communication companies (MECCs) DesignWrite, Parthenon Publishing, and Oxford Clinical Communications to work on publication plans and publications for HRT. These agencies created suites of articles and conference presentations that were intended to maintain and expand the market for HRT. Over the course of six years, DesignWrite produced for Wyeth ‘over 50 peer reviewed publications, more than 50 scientific abstracts and posters, journal supplements, internal white papers, slide kits, and symposia’.
Hormone replacement therapy has been part of a somewhat speculative, though largely successful, attempt to label menopause a condition of deficiency. Reassuring doctors and patients would turn out to be particularly important commercially, because in 2002 the routine acceptance of HRT for women was shattered. The results of the Women’s Health Initiative study indicated that women who used oestrogen plus progestin HRT faced an increased risk of breast and ovarian cancer. What is more, while it had been expected that HRT would decrease the risk of cardiovascular disease, the study suggested that the risk actually increased. After the Women’s Health Initiative definitively showed problems with hormone therapies, Wyeth’s new publication plan was called ‘Achieving Clarity, Renewing Confidence’. That effort continued previous efforts to establish confidence in the face of cancer worries: on an earlier note about breast cancer risks, a Wyeth employee had written ‘Dismiss/distract’.11
Here I will follow one of these ghostly articles, labelled PC(2) in Wyeth’s plan, on a hormone treatment with the feminine-inflected brand ‘Totelle’. The first draft of manuscript PC(2) was ready on 16 August 2002. Jean Wright, a member of the Totelle team working for the British MECC and publisher Parthenon Publishing, contacted a group of Wyeth employees. ‘Please find attached the first draft of PC(2)’, she wrote. This manuscript, based on data coming from a clinical trial of Totelle performed for Wyeth, had the unwieldy title ‘A 2-Year Comparison of the Effects of Continuous Combined Regimens of 1 mg 17β-Estradiol and Trimegestone with Regimens Containing Estradiol and Norethisterone Acetate upon Endometrial Bleeding and Safety in Postmenopausal Women’.
Just under the title of that draft was written: ‘Author: to be determined’.
Six months later, on 4 March 2003, a tracking report – we should remember that we are dealing with large corporations, so there are things like tracking reports – on articles and conference submissions to do with Totelle showed that PC(2) was making steady progress. At that point, this high-priority manuscript had been revised once by Parthenon in response to comments made on 7 December by Wyeth employee Daniele Spielmann (revision done on 3 January), a second time in response to 5 February comments by Wyeth’s Sophie Olivier (revision done on 28 February), and had yet to be revised in light of the comments of another Wyeth employee, Richie Lu, on 28 February. It was moving toward its final shape. And it was making progress, especially good progress for a manuscript that was still without an author.
By 2 April, three authors had finally appeared on the tracking report: two medical professors and Wyeth’s Daniele Spielmann. A note on the author list read ‘Need to contact’, perhaps suggesting that the first two had not yet been consulted. The fourth draft was sent to the publication team on 12 March, and the fifth on 2 July. By 6 June 2003, the manuscript had clear authors: ‘Bouchard P, Addo S, Spielmann D, and the Trimegestone 301 Study Group’, the last of those being a label for a long list of doctors who had provided patients for the Wyeth trial.
But that was not quite the end of the road for manuscript PC(2). A 27 October 2003 report revealed that in July Parthenon updated the manuscript before it was sent out to external authors for their final review, quite possibly their first opportunity to review it. An 18 August note showed that submission had been delayed during Wyeth’s signoff process. A note followed on 29 August indicating that ‘sign-off’ was nearly complete, with another on 22 September confirming that it was in the ‘final stages of sign-off at Wyeth’. But by this time the authors had changed. They had become ‘Bouchard P, De Cicco-Nardone F, Spielmann D, Garcea N, and the Trimegestone 301 Study Group’. What had happened in the meantime, and what had happened to Dr Addo? When I was trying to follow this paperwork through all of the documents, I became concerned that I might be making a mistake.
However, an email by Spielmann explained: ‘The 2 Italian authors agree with the paper and replace ADDO [who] went to our competitors’. In an earlier email, Jean Wright of Parthenon had written: ‘Please note that S. Addo has been deleted from the author list for PC(2). Daniele was doubtful whether she should be included because she now has connections with Organon’, another drug company.
In all of this, there is no indication that the external authors had any input, in contrast to the obvious and documented input from various internal actors. On 26 August 2003, for example, Wright had completed the draft on which Wyeth eventually signed off, and mentioned only that she had dealt with queries by yet another Wyeth employee.
Authors, it seems, were largely interchangeable. They were all ‘to be determined’ until the publication team thought that the manuscript was nearly ready to be sent out to a journal. At that point, Wyeth appears to have determined who the authors would be, and contacting them was added to its ‘to do’ list. Perhaps there was not much consultation even then. When Addo established ties with Organon, Wyeth no longer wanted to work with her, and simply replaced her with two other authors. It isn’t clear that she was ever notified that she had been either put on or taken off the author list.
Although a 2004 tracking report listed the manuscript as accepted in the journal Menopause, it eventually appeared in the journal Gynecological Endocrinology – perhaps that had to do with the fact that the latter journal was then published by Parthenon. On its publication, article PC(2) took its place in the marketing effort for the new formulation Totelle. Not surprisingly, it found Totelle to be an improvement over earlier hormone treatment.
Alignment on a Plan
This is what utopia looks like from an industry perspective. We have agreement and alignment on a plan, not even just a publication, a full plan, investigators on board, agencies lined up, everybody ready to play and we’re going to get this done in a timely way, in an orderly fashion, and things work like clockwork. (Ms Perez, a planner working within a pharmaceutical company)
Publication plans set out goals: an imagined orderly performance of research and rolling out of presentations and publications; then appendices give the relevant data for each of the meetings and journals to which abstracts and papers will be submitted, the audiences they reach, their impact factors, their rejection rates, and publication lead times.12 Tactical recommendations are for specific submissions, based on strategic considerations, parcelling out data for different target audiences, time and resource considerations, and the sequence in which one wants the data to roll out. Dates of submission are laid out, and dates of publication are supposed to quickly follow.
A plan may also describe other communication opportunities, such as symposia and roundtables, journal supplements, advisory board meetings, books, speaker bureau programmes and more. Though the publication plan should be a dynamic document, changeable if circumstances change, one gets the impression of a world without uncertainty, of articles written and published on schedule. And planners take pride in their efficiency: according to one presenter, the pharma company GlaxoSmithKline did a survey of sponsored publications, and compared to investigator-led publications and publications developed by people in the company’s Clinical Research department, those developed by a planning team were submitted and published much more rapidly.
Ideally, the publication planning team should be put in place early, says seminar leader Ms Peterson, ‘before too much data has gone unpublished’. The publication planning team might be formed upon proof of concept, or two years before the expected launch of the product, or at the start of Phase III trials (trials to establish efficacy and safety before the drug is approved), or when the company begins making expenditures on commercial plans. The planning team rationalizes expenditures by integrating the company’s research, scientific communication, and marketing communication strategies. It also manages knowledge flow: planner Mr Perry advises that articles from Phase I (pilot trials typically on healthy subjects) should be written early, so that Phase II (small clinical trials to guide Phase III ones) articles can refer to them; Dr Price says that the number of articles should peak at about the time that the product launches, for maximum effect. The right knowledge flow should lead to increased presence in medical understanding and in the commercial market.
Yet much planning is more ad hoc. Articles can be delayed as they are multiply revised, authors change depending on the circumstances, and where an article is published can change at the last minute – all of these happened in the case of the sample manuscript I described earlier. In addition, publication planning has to react to changing circumstances and to the demands of marketers. Here, for example, is pharma company planner Mr Powell, speaking about how marketing messages come from the top:
At the beginning of the year, we kind of have a scientific strategy for every product, saying, y’know, these are the key messages that we’re hoping to get out, depending on what clinical data we have available. We’ll look at all the points that the upper management folks would like us to try and see if we have the data to address, and then we’ll go through it point by point and try to see.13
Generating Bulk Research
A common complaint in scientific publishing is the division of research into ‘least publishable units’, and the publication of overlapping or redundant analyses. Chopping findings into least publishable units fills journals with articles that have the advantage and disadvantage of making only one point each. Academic authors are well accustomed to multiplying papers, and also to complaining about it. However, in the pharmaceutical industry each publication is part of a marketing campaign and has an expected return. The professionalization and commercialization of publishing makes a science out of the multiplication of papers.
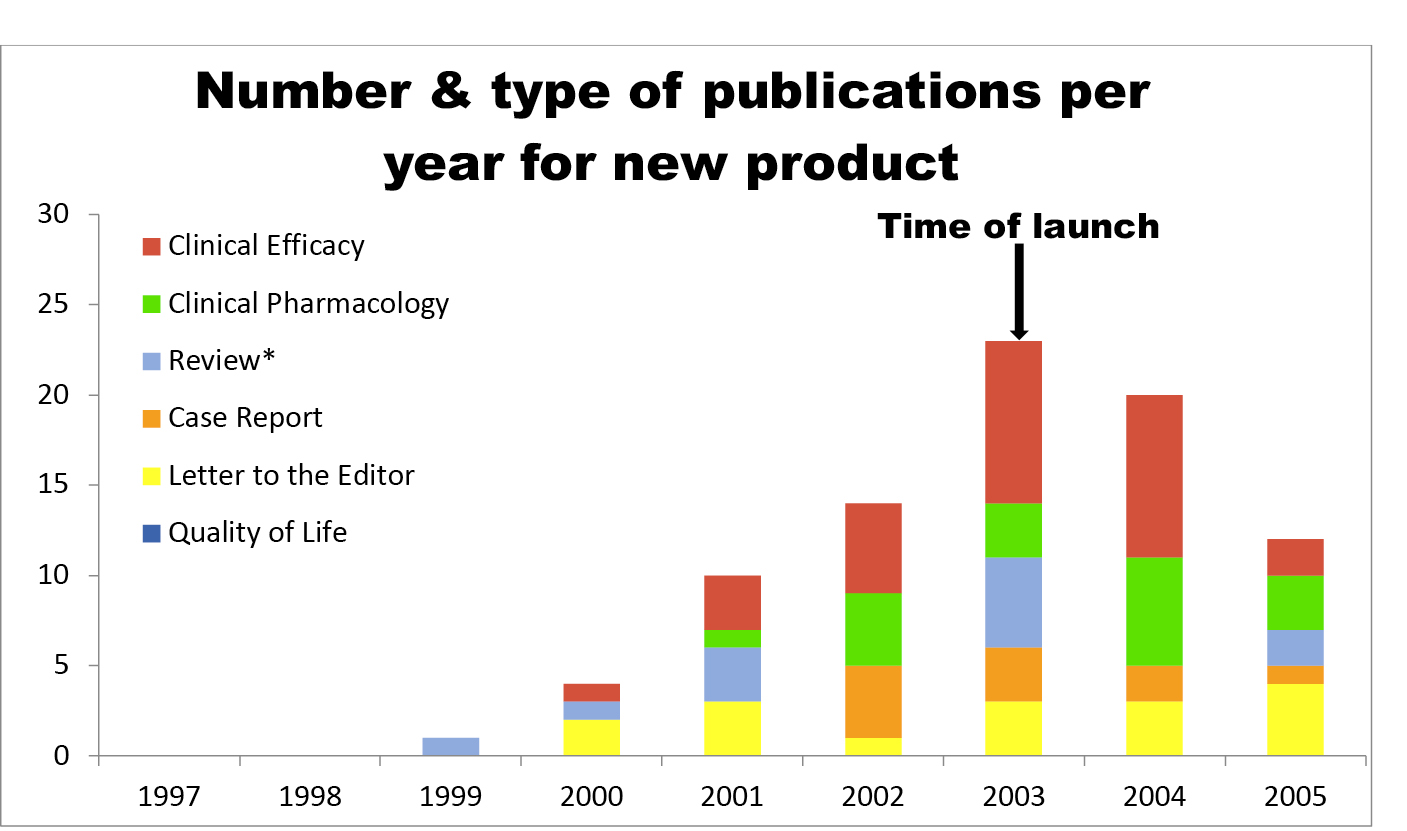
Fig. 3.1 A hypothetical trajectory of publications
There is no question that publication programmes can involve many articles. The chart in Figure 3.1, redrawn from a presentation, shows a trajectory of publications for a single fictional Product X, with approximately ninety articles in total.
I was astonished when, at an ISMPP meeting, Mr Edwards, the director of medical publishing for one of the world’s largest academic publishing companies, chided his audience: ‘You don’t help when you take your research and you do your primary publication and then you follow it with twenty, thirty, forty secondary analyses. This is alarming publication and it is actually contributing to the whole peer review process grinding to a halt.’ I am sure that he was exaggerating for effect. However, the salami slicing to which he pointed was promptly corroborated by Ms Perez, a pharma company employee who explained how to multiply articles:
There are more publication ideas coming from my medical team than we can handle even if we had fifteen agencies and twenty people focused solely on publication for this one area. That’s one of the bigger challenges, ’cause it adds more analyses. And now I need more statisticians, I need more investigators, I need more authors. I need more writers, whether they’re agency writers or external physicians doing the writing.
Perez’s eventual point was that it is important to winnow ideas early, to optimize production. She didn’t object to multiple publications, but wanted to make sure that they are all merited. There comes a point when another article isn’t cost-effective. Indeed, as another pharma-based planner observed, the bulk can become difficult to track if the manuscripts shift:
Right now our team thinks some days that we’re being a contract manager more than a publication strategist. So trying to figure out if we agreed with the author on fifteen different publications for a particular study, are we on number five, are we on number fourteen or are we approaching number sixteen and have to update the agreements? (Ms Pearson)
Marketing
Publication planners explain that their work should, though isn’t always, be independent of marketing. It should be in the service of scientific knowledge about results: ‘We really do like to stress that the publication planning company is not an advertising agency, is not a PR [public relations] agency, even though it might look like one’, says Parker. Planners understand that they are in a sensitive position. On a number of occasions, conference audience members were reminded to watch what was written down or entered into databases, because their documents and databases could become public through lawsuits or otherwise. Seminar leader Dr Price suggested, for example, that planners talk about ‘communication points’ rather than ‘messages’, because critics see the latter as driven by marketers. The Wall Street Journal in particular, with its readership in the world of finance, was mentioned fearfully several times over the course of the conference: planners want the results of their work to be reported on its pages, but not their work itself, especially work that is associated with any one drug company. Price said, ‘A publication plan might be made public, might appear on the front page of the Wall Street Journal. So you don’t want to make it appear that you don’t have authors. This is verboten today.’
In their interactions with each other and with the industry, planners recognize that their work has marketing value. Websites of publication planning firms promote their ability to market products. Envision Pharma’s website claims that ‘data generated from clinical trials programmes are the most powerful marketing tools available to a pharmaceutical company’. Watermeadow Medical advertises its mission thus: ‘Our highly qualified and insightful medical writing teams will work with you to understand your specific needs, to develop an effective and customized multichannel publication plan. Operating from principles of trust and transparency we liaise with authors, journals and internal stakeholders to translate complex scientific data into clear, clinically relevant publications’. ‘Adis Communications works in partnership with clients to position their products at the right place, at the right time’ through ‘hundreds of well-respected, and high-impact factor journals’.14
Mr Edwards, the publishing company director, explains just how crucial journals are for pharmaceutical companies. Medical journals provide registration of ideas, vehicles for dissemination, an archive of results, and certification: ‘the air of impartiality that you wouldn’t be able to get if you publish elsewhere’.
Ultimately, publication planning needs to generate revenue by providing information that increases sales. It is difficult to measure return on investment directly, says Parker, because publications typically go hand-in-hand with many other activities that affect markets and sales, as well as constantly changing markets.15 Nonetheless, one presentation by two junior planners, Ms Pham and Ms Potter, did a more direct study of return on investment for publications, by studying prescriptions of a hormone replacement therapy (HRT) by cardiologists before and after a group of published reports on HRT for hypertension, as well as patient use of HRT for the same use. After three major publications in Circulation, Menopause, and Hypertension, all showing that not only did HRT reduce the symptoms of menopause but also reduced hypertension, there was an increase in prescriptions by cardiologists, though not by gynaecologists. There were several advantages to this particular focus, including the fact that hypertension is an off-label (unapproved) indication of HRT: consequently, unless it was acting illegally, the sales force should not have been a complicating factor. Indeed, a questioner from the audience asked if the speakers themselves were doing illegal off-label promotion, an accusation they forcefully denied.
Though they appear inconsistent, planners are not merely being duplicitous when they distance themselves from marketers. They understand that their work has marketing value and is supported because of that value, but they see a clear distinction between what they do and what marketing departments do. Marketers, as planners portray them, would consistently ride roughshod over scientific standards, and would be relatively unconcerned with what the scientific data can support. To be compliant with ‘Good Publication Practice’, says Price, a publication plan is a basis for disseminating scientific and clinical data, and is ‘not a marketing communications plan’. The marketing department, Parker said, is considered lucky to have one place on a publication team – it does typically retain that one place, because ‘they’re probably paying the bill’.
Publication planning negotiates between marketing and science, implies Ms Peterson. Without it, ‘bottlenecks will inevitably occur’ and ‘vast delays are likely’, but also ‘marketing may drive the process’ and ‘the resulting publications might be “cherry picked”’. Especially in the context of scrutiny around publication of results, cherry-picking is a worry. A journal editor, Dr Ellis, corroborated the antagonism between marketing and science, exhorting her audience of publication planners to prevent marketers from writing manuscripts. She can tell, she said, when articles are written in the marketing department, and she typically rejects them; they are peppered with certain adjectives and adverbs that a scientist wouldn’t write.
Because marketers would go too far, publication planners see part of their job as constraining their influence. Yet publication plans exist to serve the marketers, and therefore the planners have to convince the marketers that their more subtle approach, with a limited range of tools, is the right one. As we’ve seen, to ‘sell without selling’ is a marketing ideal, too.16 Nonetheless, publication planning does its work almost entirely through scientific meetings and journals, without any contact with doctors.
Scientific standards are doubly important. Meeting them constitutes part of what is considered ethical behaviour, and so underpins the entire business and the distinction between doing publication planning and public relations. How, after all, could publishing high quality science be unethical? After planners persuade their sponsors that their work will provide a good return on investment, they want to obey ethical guidelines in the hands-on work they do, and to adopt high scientific standards for the writing of each article. Second, publication planners can only succeed if their work displays high standards, so that their articles will be published to best advantage. Medical journals have high rejection rates, as high as 95% in the case of such journals as the Journal of the American Medical Association and the British Medical Journal. Meanwhile, publication planners claim to have very high acceptance rates; for example, an ‘acceptance rate on first submission of 94% for abstracts and 78% for manuscripts’.17 It is only by stifling the marketing department’s efforts to hype the product that publication planners can do effective marketing to scientific audiences. At least some of the time, marketing is best done if it is invisible.
Creating Knowledge through Mediation
Publication planners are both outsiders and insiders to the clinical research world. They are outsiders because they aren’t physicians or statisticians, and don’t play a visible role in knowledge production. They are insiders because they often have detailed knowledge about clinical research, pharmacology, and medicine – in conversations I have had with planners, they have appeared fluent in the areas in which they are working. More importantly, they contribute to an enormous amount of research: a typical active planner is involved with many more research publications than are most medical researchers. Planners can, then, demonstrate expertise, though they wouldn’t normally be seen as legitimate bearers of it.
Clinical research and publication is unusual, in that acceptable methods have been very precisely spelled out, and these have been widely accepted. Reports of clinical trials are relatively formulaic and constrained, as journals demand tightly structured articles, and are increasingly demanding structured abstracts.18 Though there are many choices behind any article reporting a clinical trial, there are fewer choices about its format or language.
It may appear, then, that at least for clinical trials, the work of planners and writers is relatively mechanical, or that the work consists in balancing sponsors’ and editors’ demands, or the respective interests of marketing and science. However, designing, analysing and writing up results from clinical trials involves extensive decision-making. Planners also handle other kinds of research and manuscripts. And planners do not represent their own work as mechanical. Speaking without apparent humour, Mr Porter tries to present agency concerns to those working in pharmaceutical companies:
My plea here is to think again about attempting to commoditize something [publication planning and medical writing] that is actually a highly tailored service, a professional skill. I believe that commoditization undermines the value of medical writing. You’re not buying widgets.
Despite appearances, one cannot buy manuscripts by the gross – by the dozen perhaps, but even then they are individually crafted.
Manuscripts, and the drafts and analyses that precede them, pass through the hands of many skilled contributors and reviewers. In addition to making their own contributions, planners facilitate their teams’ work, keeping in contact with medical writers, making sure that all documents produced are consistent with the plan, managing information, and reconciling divergent demands and suggestions. The work of the planner is creative mediation, using the insights of the many people who come into contact with data and drafts to develop manuscripts that will fare better in peer review and will have an impact.
Manuscripts run a gauntlet, being subject to scrutiny by many actors. Figure 3.2 is a composite image of the people and departments involved in a publication programme, pulled together from different presentations. The number of people potentially involved is enormous, and most of them are working with or checking manuscripts to make sure that they serve the company’s interests.
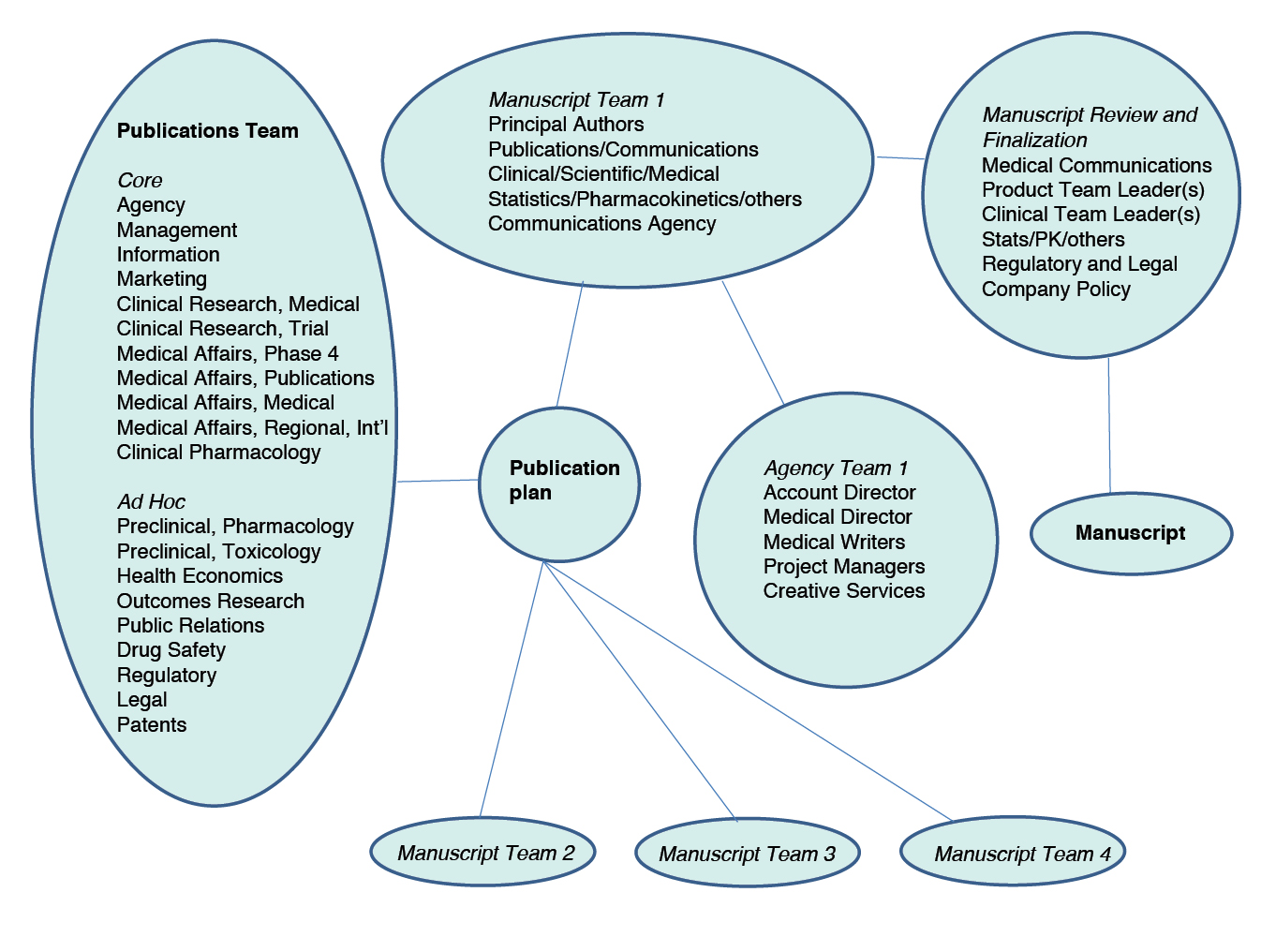
Fig. 3.2 Contributors to the publication plan and manuscripts
The overall publication planning team, says Peterson, ‘ensures buy-in from all stakeholders’ because those stakeholders have input into the process and result. The multiplication of contributors multiplies the knowledge. Pharma company planner Ms Perez says:
Involving folks early works. The sooner you involve them before you have data available the easier it is. Much, much easier. You go from having one manuscript to having eight from a pivotal programme. Which is phenomenal. And it’s not data-mining, it’s just things that are relevant to the clinical practice in that area.
Of course, many actors may give the manuscript only a cursory review, and may have little or no positive input into it. Dr Price notes, ‘All the people on the [manuscript development] team have input. But if three or four can get together and work things out’ it will be a lot more efficient. Similarly, Mr Palmer, an efficiency expert, claims that the internal review process is the most time-consuming part of producing a final manuscript, and so there are advantages in consolidating it.
Planners and the people who work with them have considerable expertise, often knowing something about their subject matter, but also having the experience of working on a much larger number of manuscripts than do most researchers, and therefore understanding the world of medical publishing very well. In addition, they sit in the middle of a number of other experts who are all interested in producing high-quality publications, and who are contributing to them, albeit in different and possibly contradictory ways. Major articles in any field involve careful rhetorical work, but ghost-managed articles are prepared by a distributed network with access to substantial resources. In the context of regimented demands from journals, and a suppression of individualized voices, science by committee works well.
A Sample Manuscript (II)
To see more concretely how the publication planning process affects publications, it is worth looking at another sample manuscript. Here, I am mostly following the careful research and analysis conducted by psychiatrists Jon Jureidini and Jay Amsterdam and philosopher Leemon McHenry, who examined a trial of an antidepressant: the CIT-MD-18 paediatric trial of the antidepressant citalopram (Celexa and other trade names) by the company Forest Laboratories. One of the most controversial topics in medicine over the past few decades has been whether doctors should prescribe antidepressants to children and adolescents. A number of drug companies have targeted this potentially huge market, identifying anti-depressants as a possible response to the difficulties that many children, and especially adolescents, routinely face. Jureidini and colleagues were working from a trove of documents available as a result of a legal case on which they served as expert witnesses.19
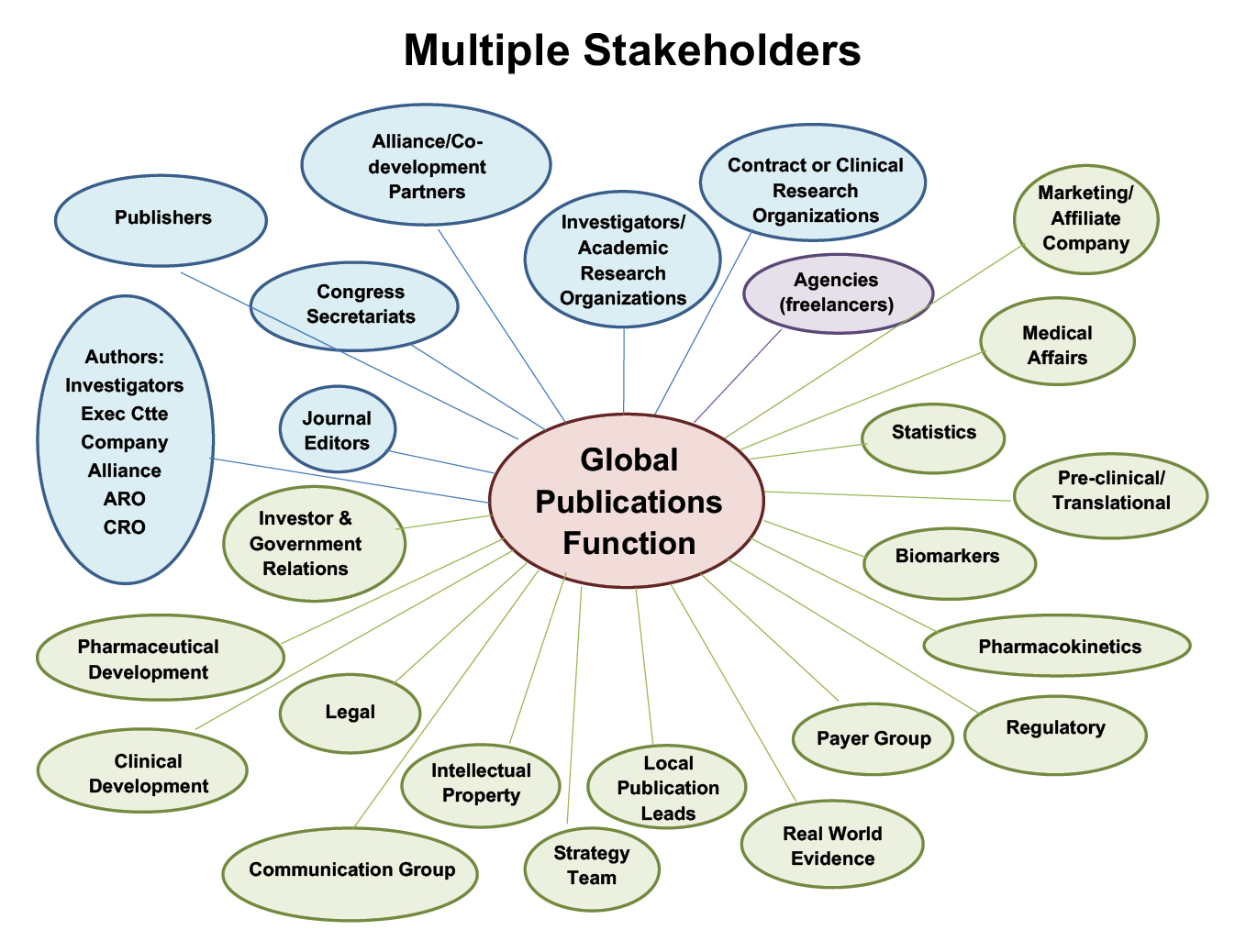
Fig. 3.3 Multiple stakeholders in publication planning, redrawn from a 2017 presentation
The CIT-MD18 trial had 174 subjects, split between the citalopram arm and the placebo arm. They were also to be split between a group aged 7-11 and one aged 12-17, to allow for comparison between these two age groups. The trial protocol was the responsibility of Forest Laboratories’ Associate Medical Director, Paul Tiseo. Therefore, Tiseo was a potential author on the eventual publication of results; as it turned out, he made a brief appearance as an author on the second draft of the manuscript, only to be dropped before it was published.
Many other people were involved. A professional writer, Natasha Mitchner, working for the agency Weber Shandwick Communications, wrote the various drafts of the manuscript. Jeffrey Lawrence, who worked in Forest’s Marketing Department, was the liaison between the company and the agency. Various other people within Forest appear to have had the power to sign off on the manuscript, or to comment on it. Again, none of them ended up as authors on the publication.
The lead author on the publication was a prominent child psychiatrist, Dr Karen Wagner, chosen because of ‘corporate objectives’. Her clinic had run one site of the trial, and Wagner advised Forest Laboratories about marketing strategy. However, Jureidini and his colleagues, who examined all of the Forest documents related to CIT-MD18 and the publication, report that they ‘could find no evidence in the extensive documents … that Dr Wagner contributed to the study design, analysis of data, or preparation of the first draft of the manuscript’. In fact, Wagner almost certainly did not contribute to the first draft of the manuscript, because at one point Lawrence asked Mitchner, the writer:
Could you do me a favour and finish up the paediatric manuscript? I know you said you only had a bit more to do. … I took a quick look at it and it looked good so I’d like to get it circulated around here before we send if off to Karen [Wagner].20
Three days later, Mitchner turned the draft in to Lawrence, referring to it as ‘the Wagner manuscript’.
The marketing department’s control was not incidental to the shaping and placing of the manuscript. In an email to Mitchner and the others, Lawrence wrote:
As you know, we don’t want to compromise the publication but we would like to wrap some PR [public relations] and CME [continuing medical education] around this data.21
Later in the same chain of emails, Christina Goetjen, one of Lawrence’s colleagues at Forest and the product manager for the drug, suggested that they change the target journal. She wanted to take quick commercial advantage of this and other studies:
I think it no longer makes sense for us to be looking at JAMA [Journal of the American Medical Association] as our publication of choice for PED [paediatric] data as the timing and policies restrict us from ‘making hay while the sun shines’.22
During the trial there was a packaging problem, and nine of the subjects were able to see that they were taking citalopram, rather than the placebo. Jureidini and colleagues, examining the document trail, argue that, according to the study protocol, the nine subjects who received unblinded versions of the drugs should have been excluded from the analysis. Indeed, Forest ran the numbers without those nine, and the result fell just short of statistical significance, at which point eight of the subjects were added back into the analysis, improving the statistics enough to make the results significant – the problem was later hidden in the submission to the FDA, in a ‘masterful stroke of euphemism’.23 The company and its communications agency made various other small late decisions to ensure that the results would look favourable. In particular, they decided not to publish the secondary outcomes. Here is Mary Prescott at Weber Shandwick Communications:
I’ve heard through the grapevine that not all the data look as great as the primary outcome data. For these reasons (speed and greater control) I think it makes sense to prepare a draft in-house that can then be provided to Karen Wagner (or whomever) for review and comments.
A number of months later, Forest’s Dr Heydorn wrote:
The publications committee discussed target journals, and recommended that the paper be submitted to the American Journal of Psychiatry as a Brief Report. The rationale for this was the following: … As a Brief Report, we feel we can avoid mentioning the lack of statistically significant positive effects at week 8 or study termination for secondary endpoints.24
And, of course, the eventual published article in the American Journal of Psychiatry did not draw attention to the serious adverse events suffered by participants in the trial.25 The decisions were crafted so as to make the resulting article as straightforwardly positive as possible, with at least most of them being justifiable in terms of one or another opportunistically chosen norm. The courts will decide whether Forest’s amount of spin in this trial exceeded the limits of ordinary scientific reporting.
Conclusions
Why should we care about the pharmaceutical industry’s ghost management of medical publications? The most common answer focuses on the possibility of fraud – or at least untruths – and that people might be harmed as a result. Philosopher Leemon McHenry argues that ‘[i]f the results of industry-sponsored clinical trials were reported honestly, then aside from the question of deception and plagiarism, ghostwriting would not present a serious concern for advancing knowledge’.26
Pharmaceutical companies and others defending themselves against accusations of ghostwriting often also try to make the issue about honesty and truth. In a statement for a news story about Wyeth’s ghost-managed hormone therapy work, Pfizer (which bought Wyeth) wrote of industry critic Adriane Fugh-Berman, ‘Even with her critical perspective, she could not establish that there were inaccuracies in any of the peer-reviewed articles’. Defending two researchers accused of serving as authors on a ghostwritten editorial, a University of Pennsylvania spokeswoman insisted that the editorial ‘notes conclusions that remain widely accepted today’. Trying to stake out the very highest ground, a founder of one MECC writes of a ghostwritten textbook in psychiatry:
the effort to produce this handbook led to a good quality project and everybody wins. The psychiatrists assure the quality, and they enhance their visibility and reputations. The writers improve clarity. The readers get good information. … For all we know, the book could have saved thousands of lives.27
While I don’t want to dismiss concerns about fraud and truth, I think that a singular focus on these concerns is the wrong approach.
Scientific knowledge is the result of much hard work: work in the lab or the field, analytic and conceptual work, work to get attention, work to convince other scientists, and much more. There is no easy and direct path from nature to knowledge of nature; if there were, then we could bypass the work. No method identifies truths with certainty; if it did, there would be a lot less disagreement in science.
We can assume that the same types of factors are at play in the production of truth as in the production of falsity. Since ideology, idiosyncrasy, interests and the like are routinely invoked to explain beliefs thought to be false, they should also be invoked to explain beliefs thought true. This is a kind of symmetry.28 Symmetry suggests that good and bad science are alike in the fact of being shaped by interests, or that good and bad science are alike in the fact of being laden with choices.
However, commercially driven science is different from academic science in the kinds of interests that drive it, and the kinds of choices it contains. Commercial funding and control affect a myriad of legitimate choices in the design, implementation, analysis, description, and publication of clinical trials. We can reasonably expect, and there is abundant evidence, that the industry makes those choices to support its interests. The good science, and not just the bad science, supports pharma’s interests.
Ghost-managed work succeeds by being of apparent high quality. Publication planning firms claim high acceptance rates for the articles they submit to medical journals; high acceptance rates are very credible, though we should suspect that all individual claims are exaggerated. Industry-funded trials – most of which I would claim are ghost-managed to some extent – score as well as or better than independent trials on standardized methodological tests.29 Moreover, when I observed publication planners, they appeared to be trying to be honest and to be striving for sound science, while serving the interests of drug companies’ marketing departments. So, pharma’s ghost-managed science looks like its counterparts, and even looks particularly clean and successful in comparison. The industry does not have a monopoly on fraud and misrepresentation – as can be seen by taking a quick look at the website Retraction Watch, which reports daily on problems in published scientific articles.30 The industry sometimes gleefully latches onto instances of misconduct in which it isn’t involved, or in which it isn’t the perpetrator. At times, pharma even assumes the role of victim, as when it was revealed that Dr Scott Reuben faked the results of twenty-one trials, many of them funded by the drug company Pfizer.31
From the cases of pharmaceutical industry misrepresentation, we know that at least some of the time publication planners and the others with whom they work fail in or set aside their goals of honesty and sound science. But we should also be concerned about what happens the rest of the time.
Ghost management produces a publication bias that covertly advertises particular drugs, supports them scientifically, and sets agendas for diagnosis and treatment. All of this affects prescriptions. Agenda setting is particularly important, as it can dramatically increase the number of patients seen to have a given disorder, and can dramatically increase the number of patients seen to need treatment; as drug-taking populations increase, so will rates of side effects. To the extent that all the above are different effects from those of a non-commercial scientific literature, they probably harm patients – without requiring any misrepresentation of data.
In the ghost management of medical research by pharmaceutical companies, we have a novel model of science. This is corporate science, done by many unseen workers, performed for marketing purposes, and drawing its authority from traditional academic science. The high commercial stakes mean that all of the parties connected with this new science can find reasons or be induced to participate, support, and steadily normalize it. It is likely to be around for a while.
We might then ask the widely circulating joke, ‘is medical science for sale?’ ‘No, its current owners are perfectly happy with it.’