7
Sirens of Hope, Trolls of Fury and Other Vocal Creatures
A Satisfying Encounter at the FDA
The year 2015 witnessed a notable victory for pharma-crafted patient advocacy. That year, Sprout Pharmaceuticals resubmitted flibanserin, with the commercial name Addyi, to the US Food and Drug Administration (FDA) for market approval. This was the third attempt through the process for flibanserin, intended to treat female sexual dysfunction. Despite the fact that the FDA’s advisory committee had originally voted eleven to zero against the application, and despite the fact that the FDA had concerns about both the efficacy and safety data supporting the application, the agency approved the drug on this third try. Within two days, the larger company Valeant Pharmaceuticals snapped up little Sprout and the drug for one billion US dollars.
The submitted trials showed that women on flibanserin had only 0.5 more satisfying sexual events per month than women on the placebo. Because the trials excluded women with even mild depression and anxiety, the FDA wasn’t convinced that the safety data was adequate. In fact, women on the drug had higher levels of sleepiness and sedation than had women on the placebo, and drinking alcohol while on flibanserin was connected to dangerous drops in blood pressure.1
The difference between the FDA’s first and last decision on flibanserin was almost entirely due to Sprout’s aggressive public relations campaign, ‘Even the Score’. Even the Score put the blame for the lack of female sexual dysfunction drugs on sexism, and put pressure on the FDA to approve flibanserin as a matter of women’s equality. One of the central designers of the campaign knew her target: Audrey Sheppard had joined Sprout shortly after having served as the head of the Office of Women’s Health at the FDA.2 The campaign involved an extensive online presence on Twitter, Facebook and other platforms, involving such things as parodies of Viagra ads: ‘What the fuck?’, asks a woman in one such ad. ‘Are we really so far behind we don’t think women have the right to sexual desire? Yet again we come second.’3 The campaign also gathered a number of important partners, including the National Organization for Women, the Black Women’s Health Imperative, and many other national women’s organizations in the US. For the National Consumers League, Addyi was ‘the biggest breakthrough for women’s sexual health since the pill’, and other organizations made similarly expansive statements.4
When the FDA held public hearings on the drug, scores of women showed up to make the case for this ‘pink Viagra’. Many were ‘carrying gift bags, matching scarves, and large buttons with the “Even the Score” campaign slogan’, a not-so-subtle sign of their having been been recruited and bussed in by the company.5 Indeed, disclosures showed that the expenses of many of the women had been paid by Sprout, directly or through an intermediary, Veritas Meeting Solutions; a number of them also shared a urologist, Irwin Goldstein, a Sprout-connected KOL who had recruited them for the FDA meeting. As reported by Judy Segal, a scholar of the rhetoric of science who attended the meeting, some speakers appeared to be ‘ventriloquized’ by Sprout. Says one,
I think the thing that makes me most angry and most disappointed is that if I went to my doctor and I was a man and I said these things they would be able to write me a prescription within a couple of minutes for a drug that is insurance covered and FDA approved.6
Moreover, ‘most of the testimony the FDA would hear came from married women who had no interest in sex with their husbands and felt themselves to have a biological disease that was, moreover, threatening their marriages. Eight women testified; six of them told deeply personal stories that ended with an emotional call for drugs.’7 The company had developed effective patient advocates.
Despite its eventual success at the FDA and for the owners of Sprout, Addyi has not been a sales success so far, with some insurers declining to cover it. This may be because it’s an expensive pill taken daily, requires its users to abstain from drinking alcohol, and offers only modest rewards. ‘Where’, ask commentators from the organization PharmedOut, ‘are the crowds of women with low libido clamouring for Addyi? They never existed, except in a PR firm’s fantasy.’ But that PR firm’s fantasy was rich enough to get the drug past a key gatekeeper.8
Leveraging Patient Advocacy Organizations
For the pharmaceutical industry, patient advocates and patient advocacy organizations (PAOs) are excellent spokespeople and potential allies. They are and represent key stakeholders in markets for drugs. More importantly, they are recognized as stakeholders by government regulators and insurers, and are often seen as important independent voices in public spheres. PAOs are thus perfect candidates to be phantom hands for the industry.
The idea of stakeholders has gained some importance in the industry. ‘Stakeholder relations’ and ‘stakeholder engagement’ are recent industry catch-phrases, ways of talking about the diverse work of assemblage marketing. Mat Phillips, co-founder of Engage Health Alliance – Europe, a ‘multi-stakeholder engagement organization’, insists that all stakeholders should be ‘aligned’ to ‘ensure innovation delivers the fullest value possible to those who can benefit’.9 In assemblage marketing, pharmaceutical companies often treat all the different actors as stakeholders, but patients and the PAOs that represent them are the most obvious stakeholders and carry the most legitimacy. Because of this, companies befriend existing advocates and organizations and try to build relationships that can be used whenever and wherever independent patient voices will have value.
Patient advocacy has become especially important over the past thirty years. Though effective PAOs already existed in the mid-twentieth century, it was the successes of AIDS activist groups at shaping research that charted the path for organizations focused on many other diseases and conditions.10 PAOs can do many things, including raise public awareness, promote or oppose the medicalization of conditions, voice demand for particular treatments, advocate for research, shape or engage in research programmes, provide research and other funding, provide access to patients, advocate for relevant legislation, and more.11 With only a little work, pharma can often align PAOs’ interests and activities with its own.
The EveryLife Foundation for Rare Diseases runs an annual conference – the Rare Disease Legislative Advocates conference – in Washington, DC. The event provides patients and advocates with a day of training, where they learn how to make their organizations stronger, how to have successful meetings with politicians and others, and ‘how to tell their stories’. After that, the participants go to the US Congress for a ‘Lobby Day’. They meet with congressional staffers and legislators, to press cases for funding or for particular laws. Everything is organized by EveryLife. According to Dr Emil Kakkis, EveryLife’s president, the foundation doesn’t ‘tell patients what to do on the Hill. They are given options.’12
EveryLife can provide travel grants for 100 of the 300 participants, thanks mostly to the generosity of pharmaceutical companies. In fact, the EveryLife Foundation itself is a creature of the pharmaceutical industry, receiving donations, some of them substantial, from two dozen companies. Kakkis himself is the founder of Ultragenyx Pharmaceutical, a small company that looks for treatments for rare diseases.13
I saw a more modest version of this approach at the Drug Information Association (DIA) meeting I went to in Vienna. The DIA is an association for contract research organizations, regulatory support and connected agencies involved in drug approvals. The DIA has a ‘patient fellowship’ programme that pays for a dozen or more patient advocates to attend its annual meetings each year. The programme’s published goals include improving ‘alliances between patient groups and other health care stakeholders’.14 As the programme was explained to me, the patient advocates who win fellowships tend to be relatively new to advocacy and tend to represent people with relatively uncommon diseases. Like participants at the EveryLife conference, they are treated generously, being invited to speak about their work on panels and at specially created media events, encouraged to attend sessions at the DIA that can help them develop insights and skills for successful advocacy, and introduced to potentially valuable contacts in the private and public sectors. Representatives from the fellowship programme refer to past fellows as ‘graduates’, as if they had attended a course,15 and one such graduate, describing her very positive experience, clearly portrays herself as a novice student.16 ‘Engaging and partnering with emerging stakeholders has become a crucial pharma priority’, writes a columnist in the online magazine eyeforpharma.17
In a 2010 article in the DIA’s member magazine Global Forum, Amber Spier and David Golub, both of whom work for a major consulting firm, provide an overview of ways in which PAOs can be ‘leveraged’ by the industry. They want to make a ‘compelling case for engaging advocates well before a product comes to market’.18
Engaging PAOs does not mean simply supporting them with funding. In their short case study, Spier and Golub describe how their firm convened a working group of PAO representatives before Phase III trials, in much the same way that we saw companies convene advisory boards of key opinion leaders (KOLs). Working groups can gather important market and medical information, but they’re also useful for ‘forging durable links with these key customers’. Quoting Spier and Golub, the networked patient advocates can then:
- help with many details in the design, execution and communication of clinical trials
- provide input into relevant regulatory processes
- connect companies with valuable KOLs
- offer understandings of ‘market dynamics’
- help design drug adherence and disease management programs
- influence policies, public decisions and treatment guidelines
- provide testimony to and share personal experience with regulators and other government bodies
Spier and Golub sum up the influence of PAOs in the following graphic (Fig. 7.1), which echoes several images presented in earlier chapters. Like medical publications and like KOLs, patient advocates are co-opted into the marketing process.
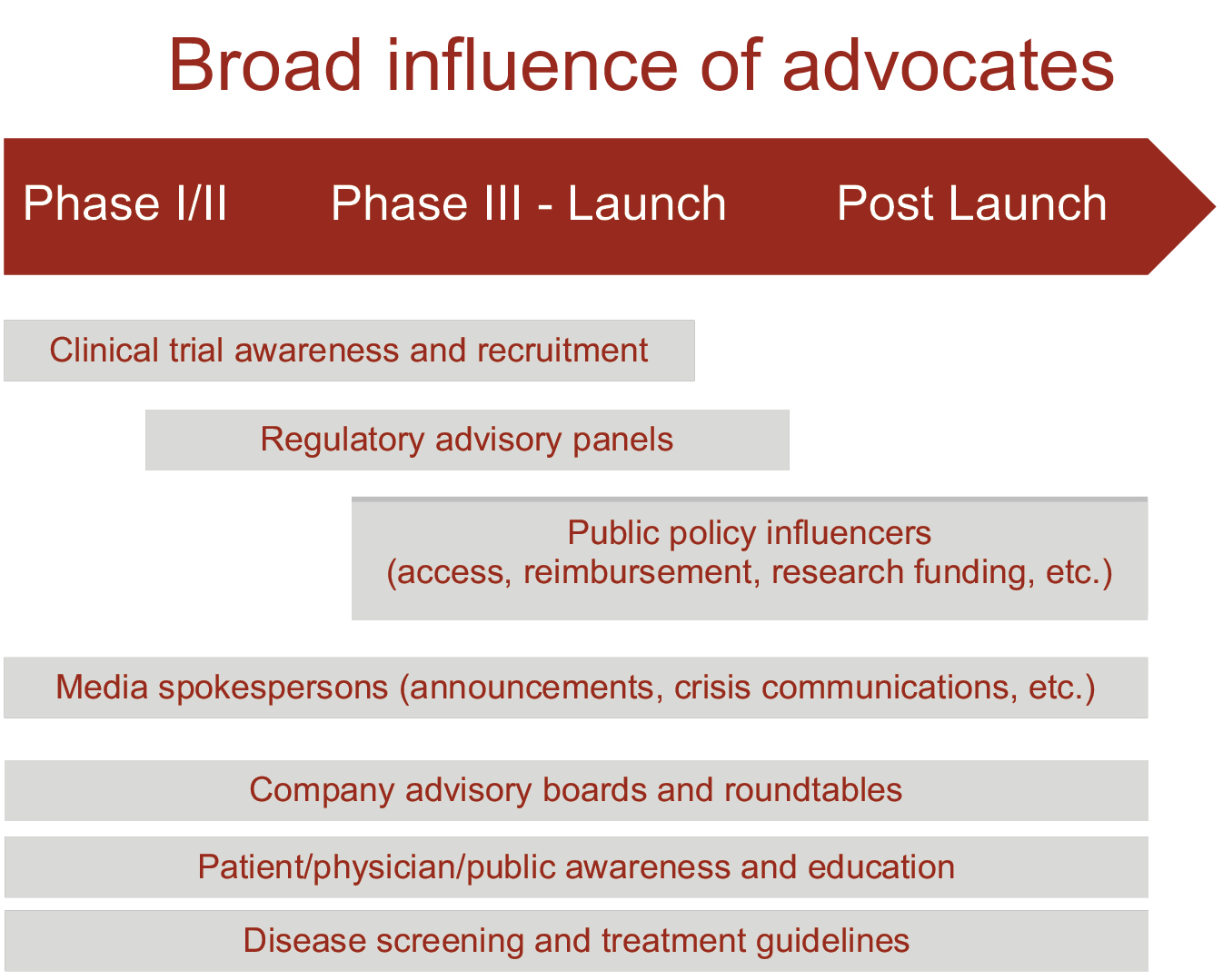
Fig. 7.1 The influence of advocates19
Established PAOs themselves often welcome partnerships. In her book Health Advocacy, Inc., Sharon Batt chronicles changes in Canadian breast cancer PAOs between the early 1990s and the late 2000s. Batt was a co-founder of one such organization in the 1990s, and uses her extensive knowledge of the terrain and the people involved to understand the changes over the following decades. At the beginning of that period, there was some Canadian government funding for PAOs, but little industry funding. The 1990s saw reductions in opportunities for public funding, followed by a vocal split within the community of cancer patient advocates about whether or not they should work with pharmaceutical companies. Those in favour of industry funding often had the rhetorical upper hand, arguing that relationships with companies ‘foster the values of trust, collaboration, information sharing, horizontality, networking, negotiation, consensus and flexibility’. In addition, PAOs that accepted government funding could be seen as having the more important conflicts of interest, because they were in a poor position to criticize government policies. In the end, industry funding became the norm.20
In 2007 the Canadian Breast Cancer Network sponsored a survey about the risk of relapse, finding that only one in ten women were aware of their risk of relapse after five years of treatment with tamoxifen, a treatment for some specific breast cancers. The Network produced a press release, an information fact sheet, and a slick video on YouTube. Batt, who followed the Network’s work for her study of breast cancer PAOs, recounts that the ‘professionalism of the package was striking and had all the hallmarks of a help-seeking ad’. Seen as an advertisement, it would have been precisely targeted, because the package was circulated through the Network’s members and contacts, who would have had reason to pay attention to a risk of relapse of breast cancer. The entire project was paid for by Novartis, which makes a drug specifically for follow-up therapy after five years of treatment with tamoxifen!21 This was the Network doing something within its mandate, by sharing useful information. At the same time, Novartis was spreading precisely the information it most cared about to its own target audience.
Allies to Transmit Hope
As in the case of flibanserin, many clinical trial results are not, by themselves, strong evidence for a drug’s value. Hope can transform weak or equivocal data into something more medically meaningful. Patient voices, often collected, articulated and amplified through patient advocacy organizations, are the most important conduits of hope, especially for regulators, but also for medical researchers. PAOs can challenge the ‘cold guardians of the public purse’, and in the process can change the meaning of data.22
Many PAOs are in the business of building on hope. They advocate in the hope of better treatments, both in terms of improved drugs and other interventions, and in terms of access. PAOs’ public faces, especially in their advertisements, appeals and websites, often present hopes in terms of perfect cures and solutions, medical ‘magic bullets’23: ‘Your gift today will help us find a cure tomorrow’, ‘Change the future’, ‘End [disease X] now’. Other PAOs channel hope differently, trying to make existing treatments more widely available: ‘When caught early, [disease Y] is highly curable’.
Government agencies listen to patients and PAOs – it is widely accepted that patients should have input into the processes of drug and other health regulation, and it is in the mandates of most regulatory agencies to listen.24 By themselves, PAOs’ pleas may not be enough to get regulators to act in one way or another. However, they can make the plight of patients more urgent and the hope contained in treatments more salient. They can make poor or equivocal data more adequate and may allow, or even convince, regulators to support the drugs at issue.
We can see some of the value to the industry of alliances with PAOs in a detailed story told by Ms Laird, who was promoting a ‘stakeholder’ approach to marketing at a pharmaceutical conference, as part of her consulting company’s approach. After describing a client company’s investment of £50 million in ‘translational medicine’ to engage with patient advocacy groups and other stakeholders operating in Scotland, Ms Laird told the following story to establish the importance of the investment, and the interactions it created:
We had a negative decision on approval of a drug for the Scottish Medicines Consortium, which is the Scottish equivalent to NICE, and the negative decision there would impact on the NICE decision which was going to happen six months down the line.
NICE is the UK’s National Institute for Health and Care Excellence, which, among other things, assesses the cost-effectiveness of drugs for the National Health Service. NICE decisions can make or break a drug in the UK.
The data was robust, there is nothing more we could have done with the data. And no other studies, no more data was required. It was absolutely, the data couldn’t have been stronger. … It was rejected on cost.
It didn’t extend life, and when they look at QALYs [Quality Adjusted Life Years], life outcomes, does it tend to excel [sic] the patient’s life? … And that is not what this product did. So the outcome of the decision was no reimbursement in Scotland. As you can imagine that wasn’t a very good day in the office.
We had four weeks to overturn or to do something about the decision before it went live on their website. And this is why it is important that you think outside the box and you work with external stakeholders. We approached six key stakeholders. … Now all the of [these] wrote to the SMC [Scottish Medicines Consortium] on their own behalf, but it was on patient choice and dignity to the patient.
Laird’s firm had earlier supported and established good relations with all of the six chosen stakeholders, but in her presentation she insisted that they all lobbied primarily because they had seen the drug’s effects, directly or indirectly.
They went way, way over and above what we asked them for, asked them to do. It was all about because they’d experienced it, they’d seen the effect that the drug had had for the patient.
And the SMC overturned the negative decision! Like I said, it wasn’t to do with the science, the science got us so far. And if we had ignored everything else then it would have been the same negative decision. So just to show you that the right result was achieved for the patient. And the learning is obviously that … this would not often have been achieved without this networking, without bringing all the organizations with us along the journey.
These guys were with us from before launch, pre-launch and they knew exactly what we were trying to achieve, they knew the outcome of the patients, they had seen it, the nurses had seen it but seeing the results they knew where we were. … No matter how good your data, you need to plan and take other people with you. Because if we had approached them with four weeks to go we would not have got the result for the patient, because they wouldn’t have been with us along the journey, they wouldn’t have understood the science, the data, they wouldn’t have seen the patient experience.
Of course, the SMC had seen data on the patient experience, but wasn’t initially convinced that that data made a strong case for the drug. In Ms Laird’s words, in terms of ‘QALYs, life outcomes, does it tend to excel [sic] the patient’s life? … And that is not what this product did.’ The drug’s benefits were more intangible. But when prodded by the company, these patient organizations felt able to appreciate them, no doubt on the basis of their earlier good relations with the company. Focused hope is one of a drug’s most valuable ingredients.
Public Relations in Echo Chambers
In the US, at least five-sixths of the hundred largest PAOs, and two-thirds of all PAOs, receive funding from the pharma or medical devices industry; 12% receive more than half of their funding from those industries.25 At the same time, these organizations are very unlikely to report industry funding: one study compared the grant registry for Eli Lilly and the disclosures of all the PAOs on that registry; only a quarter of them acknowledged the funding.26
When the FDA invited select groups to hearings about new rules for evidence to speed up drug applications, thirty-nine of the forty-two PAOs had received funding from drug companies, and at least fifteen of those had pharmaceutical or biotech executives on their governing boards. A reporter remarked that the most eloquent speaker in these hearings was Marc Boutin, CEO of the National Health Council, ‘a united voice for people with chronic disease and disabilities’. But not only does the Council receive 77% of its funding from pharmaceutical and biotech companies, but those companies are well represented on its board of directors and its key committees.27
When the European Medicines Agency (EMA) proposed in 2012 that all the clinical trial data submitted to it in drug applications should be made public, the pharmaceutical industry went into high gear. As we’ve seen, clinical trial results submitted to regulators often provide only weak evidence for drugs’ effectiveness and safety. A 2013 leaked email from the European Federation of Pharmaceutical Industries and Associations to a long list of drug companies set out a four-pronged campaign to oppose the EMA’s move. The first step was ‘mobilising patient groups to express concern about the risk to public health by non-scientific re-use of data’. (The other three prongs of the campaign involved creating other alliances: convincing scientific associations of the dangers of data transparency, recruiting allies from other industries that might be concerned about trade secrets, and creating a network of KOLs ready to counter specific interpretations of data.) But why would PAOs be opposed to transparency? And why would they be opposed to transparency of results submitted in drug applications and not, for example, all the results published in medical journals?28
When Novartis challenged India’s patent law over the decision not to grant a patent on the anti-cancer drug Glivec, the mobilization of PAOs was crucial on both sides. The company had been denied a patent on the grounds that the drug was a mere tweaking of an earlier one that wasn’t protected in India. The case became symbolically important in fights around globalization, between competing pharmaceutical industries and over healthcare. As a result, the alliances on both sides involved PAOs. Through a programme that provided Glivec for free to some low-income patients, Novartis recruited patient voices in the political battle. Meanwhile, the central organizations in the anti-Novartis alliance included the Cancer Patients Aid Association and the Indian pharmaceutical industry, which manufactured generic versions of Glivec. Although Novartis lost its Indian court case, its programme to selectively give Glivec away won public relations wars elsewhere, and this was the company’s prime concern.29
Conflicted PAOs and spokespeople talk to regulatory agencies that are themselves often rife with conflicts of interest. Employees of national regulators routinely move from government to industry and back again. People working at the highest levels of government, setting policy profoundly affecting pharma, also walk through those same revolving doors. In the UK, within six months of stepping down from his position as CEO of GlaxoSmithKline, Andrew Witty was asked to head the Accelerated Access Review programme, which is tasked with bringing ‘innovative’ treatments to patients more quickly – something that benefits pharma.30 Two months before he left his position as Executive Director of the European Medicines Agency, Thomas Lönngren set up a consultancy within a company that helps pharma companies get drugs approved.31 The former President of Eli Lilly, Alex Azar, is, at the time of writing, Secretary of the Department of Health and Human Services in the US.32 Meanwhile, the Commissioner of the US Food and Drug Agency is Scott Gottlieb, a venture capitalist who has served on the boards of various pharma companies.
On 1 September 2017, the prominent health newsletter STAT News published an op-ed by Dr Robert Yapundich, entitled ‘How Pharma Sales Reps Help Me Be a More Up-to-Date Doctor’.33 Yapundich, a neurologist who has been in practice for more than twenty years, argued that sales reps should be allowed to discuss ‘off-label’ uses of drugs – uses for which the drugs aren’t approved. This, he said, drawing on anecdotes about patients, would allow him to better help his patients. Yapundich’s bio mentioned that he was a member of a US group called the Alliance for Patient Access. Another newsletter, HealthNewsReview, quickly pointed out that Yapundich had accepted a considerable amount – more than $300,000, as it turned out – from the drug industry in recent years, and hadn’t noted the conflict of interest. Embarrassed by these and other revelations, STAT News withdrew the article.34
Although the name Alliance for Patient Access suggests a patient organization, it is officially an organization of physicians. The physicians who sit on its executive include some of the industry’s most highly paid KOLs, including Dr Srinivas Nalamach, who received $800,000 from drug companies between 2013 and 2015, in connection with the promotion of opioids and drugs to treat the side effects of opioids.35
Yapundich had not reported his conflicts of interest, but more importantly, he had neglected to mention that the article was drafted for him by a public relations firm. Yapundich stood by the article, though he acknowledged that the ghostwriters had either fabricated or made mistakes about some details of the anecdotes.36
That’s not all. The Alliance is supported primarily by membership dues paid by pharma companies and trade associations, and is operated by the public relations firm that commissioned the ghostwritten op-ed. So, what is apparently a patient organization is officially a physician organization that is actually a pharmaceutical industry organization – or a creature of the industry.
It is less of a paradox, then, that the Alliance for Patient Access opposes limits on drug costs, even though high costs clearly affect patients’ access to drugs.37 Strong patents create monopolies that allow for very high prices. Nonetheless, there is no shortage of PAOs willing to advocate in favour of patent protections for pharmaceuticals in the name of increased innovation. In response to discussions on a United Nations panel that pointed the finger at drug patents as key culprits in maintaining high prices for much-needed drugs, thereby keeping them out of the hands of patients, fifty PAOs wrote to then-Secretary of State John Kerry, to support the US government’s strong defence of the patent system. Some of those PAOs might have been acting out of hope for magic bullets, and some might have been acting purely as creatures of the pharmaceutical industry. The Global Alliance for Patient Access, a spin-off project of the US Alliance for Patient Access, was one of the signatories.38
Among all these PAOs with similar names, and which invoke similar high-minded principles, are some that genuinely advocate for public access and affordability.39 But there are just as many that are deeply conflicted. The Centre for Medicine in the Public Interest, operating in the no-holds-barred arena of US politics, is one of the most blunt and troll-like of all the PAOs supporting the pharmaceutical industry’s interests. It describes itself as a ‘non-profit, non-partisan organization promoting innovative solutions that advance medical progress, reduce health disparities, extend life and make health care more affordable, preventive and patient-centred’. To illustrate just how aggressive the Centre can get, a column on its website awarded a ‘Pharma Idiocy Award’ to two Yale professors, Cary Gross and Abbe Gluck, for their editorial on the ‘Soaring Cost of Cancer Treatment’. Robert Goldberg, writing for the Centre, alleged that the ‘authors managed to synthesize every pedestrian and inchoate assault on drug companies into an editorial that took the genre to a new level’. Goldberg writes:
The failing heart of the article … can be obtained by reading one paragraph: (I am sparing you the painful waste of time required to slog through the entire article and endure the smell of decomposing bromides).
He then quotes what he takes to be the most offensive few sentences from the article in question. Here are Gross and Gluck, quoted by Goldberg:
We know that the cost of cancer drugs has increased dramatically, even though most drugs are brought into the market without compelling evidence that they prolong survival or improve quality of life. We know that these high costs render state-of-the-art cancer treatment unaffordable to patients without insurance and even to some patients with insurance. Furthermore, financial distress associated with paying for cancer treatment is common and is associated with stress, decreased adherence, bankruptcy, and worse outcomes. Finally, we know that the cost of new drugs is not well correlated with their effectiveness, nor with the presence of competing products.40
Gross and Gluck’s words appear unexceptional. However, Goldberg takes aggressive exception to their view:
I won’t take on every citation Gross and Gluck (Gross-Gluck sounds like a Borscht Belt act) use to assert perfect knowledge about the havoc price increases have had on society.
Goldberg concludes by calling the article a ‘half-baked convoluted diatribe’, and calls ‘[s]hame on the medical journals that continue to publish [such] anti-pharma crap’.41
Advocates and PAOs can do important work to distract attention from the costs of drugs – with more subtlety than the Centre can muster. Almost every time that PAOs call for more support of innovation, they echo pharma companies’ refrain that high prices are necessary to bring new drugs to the market. Much of the time they point the finger at insurers (public and private) for not covering all drugs, in an attempt to deflect attention away from pharma.
Promoting Diseases and Treatments
In 2016, a public relations company, CGI Group, sent out a press release inviting prominent Canadian newspapers and broadcasters to interview a well-known Canadian comedian, Cathy Jones, on the topic of ‘vaginal atrophy’. The Toronto Globe and Mail took Jones up on the interview, and ran the story. As a comedian, Jones was comfortable having a light-hearted discussion about what could have been an uncomfortable topic.42
There wasn’t any mention that Jones was being paid to do these interviews. Nor was it made clear whether she had or didn’t have vaginal atrophy – as she puts it, she was just trying to convince women to talk to their doctors, because she feels ‘passionate about vaginal health’. There wasn’t any mention of drugs, or of the drug company paying for the PR campaign. ‘No parties including GCI want any mention of the drug or drug company’, a contact for CGI told the Canadian Broadcasting Corporation in one of its pitches for an interview. ‘It’s an unbranded campaign.’43
‘Vaginal atrophy’ is a recently developed name for a loose collection of symptoms, including dryness, itching, burning and soreness. Most of the prominent medical science publications that mention vaginal atrophy are either sponsored by or are based on the direct research of one drug company, Novo Nordisk. These publications tend to prominently feature local oestrogen therapy, a treatment manufactured by, of course, Novo Nordisk. In 2007, The North American Menopause Society published a positive statement on local oestrogen treatment for vaginal atrophy. That statement, too, was supported by Novo Nordisk, and it was turned into a continuing medical education course for doctors.
For a condition like vaginal atrophy, it’s valuable to have patients approach their doctors to seek treatment. Therefore, Novo Nordisk wants to get both patients and doctors using its preferred way of understanding symptoms, and even its preferred term. To that end, it does things like hire PR firms to have stories about the condition planted in the media, featuring ‘patient advocates’ like Cathy Jones. CGI is right that it’s an ‘unbranded campaign’: unbranded in the sense that the official brand is lurking in the murky background. But the unofficial brand is the term ‘vaginal atrophy’ itself, and that is firmly front and centre.
The vaginal atrophy campaign was a broad one, intended to reach many readers of newspapers and viewers of television. Especially in countries like Canada, which partially restrict direct-to-consumer advertising of drugs, companies find it valuable to use both broad and narrow campaigns.
Conclusion: Arranging the Chorus of Patient Advocates
I began this chapter with the case of the approval of flibanserin, the drug for female sexual dysfunction. The company owning the drug worked with women who were diagnosed with the dysfunction, and developed individual advocates to help make the case that a treatment was urgently needed. This is just one of an increasing number of similar stories, especially for drugs for rare diseases – a growth area for the pharmaceutical industry. The advocates were acting in what they saw as the women’s best interests, which was also in the company’s best interests.
If companies can bring patient advocates onside, they can use those advocates in a variety of ways and for a variety of ends. They can articulate need, urgency and hope where it can make a difference. In addition to intervening with regulatory bodies, advocates can influence policy, serve as conduits of information, act as spokespeople for public relations campaigns, and promote treatments and diseases to other patients. When they need to, companies can create patient advocates and advocacy organizations out of thin air (and money), to give voice to their interests in a way that has or can be taken to have legitimacy.
Carefully engaging sirens of hope can make the difference between a molecule and a profitable drug. Ventriloquizing the occasional troll to beat the drum for companies’ interests and to silence critics can make for a profitable environment more generally.