1
Power and Knowledge in Drug Marketing
Pharmaceutical Company Interventions
Drugs are at the centre of medicine. Most medical scientists do research on the effectiveness and safety of drugs. Most physicians working in modern scientific medicine focus on solving problems by providing drugs. The same is true of most patients: when we walk into the doctor’s office, we usually hope to walk out with a prescription for drugs that promise to heal us, to improve our quality of life, or to keep us in good health. But which prescription? Why that one? What leads our doctor to write those particular words and symbols on her little square of paper?
Imagine this scenario: after seeing a TV ad for some drug (perhaps you can’t quite remember which), you think that it might be time to get your cholesterol checked. Your doctor agrees, saying that adults should have their cholesterol checked every five years, and you head down to the lab. The results come back, and you learn that you have somewhat elevated LDL cholesterol levels, although not enough to panic. But considering that you’re firmly in middle age and had an apparently healthy uncle who had a heart attack at 70, your doctor recommends that you take a statin. You start to ask a question, and he interrupts: ‘These drugs are so safe they should be added to the drinking water.’ There are various choices, but he recommends Zovachor [not a real drug name], one of the biggest sellers. He’s been prescribing it for years, and he has just read an article that showed that, for people in your age group, Zovachor had the best benefit-to-risk profile of the major statins. He had heard one of his old medical school profs speak about it at a conference he attended last year, and that guy practically wrote the book on heart disease. He hands you a free sample, and a prescription. You leave, feeling safer.
How many times might drug companies have intervened in this scenario? Of course, a company placed the ad that convinced you to see your doctor. That’s One. Should you have taken the test? What is an elevated cholesterol level? Drug companies have helped to fund research that has resulted in recommendations for regular testing and that has steadily lowered what physicians consider normal cholesterol. Two and Three. They’ve also funded the studies that identify risk factors like your uncle. Four. Who did the safety studies on statins, and years later have still not released all of the data? Five. Who has promoted the slogan ‘so safe they should be added to the drinking water’, which almost every doctor has heard? Six. Your doctor was probably given that article on Zovachor by a drug company sales rep. Seven. Chances are that the article itself was ghostwritten for the maker of the drug, given to some highly regarded professors of medicine to put their names on, and then submitted to a good medical journal. Eight, Nine, Ten. Your doctor was probably funded to attend that conference. Eleven. His former professor was also probably funded, and another company ghostwriter may have written his talk. Twelve and Thirteen. In fact, that professor’s reputation as a whole has almost certainly depended on research and publication help from the industry at many stages. Fourteen. And then there’s that sample, placed in your doctor’s cabinet by a sales rep the week before, encouraging him to prescribe the drug. Fifteen, and counting.
Ghost-Managed Medicine is a study of the pharmaceutical industry and its agents, as they try to shape and spread the medical knowledge of value to themselves.
Pharmaceutical companies sustain large networks to gather, create, control and disseminate information. They provide the pathways that carry this information, and the energy that makes it move. Through bottlenecks and around curves, knowledge is created, given shape by the channels it navigates. Pharma companies create medical knowledge and move it to where it is most useful; much of it is perfectly ordinary knowledge that happens to support their marketing goals. But because of the companies’ resources, their interests and their levels of control, they become key shapers of almost all medical terrains.
In this book, I shine a light on some important tactics and practices that drug companies use to influence medicine. I describe paths of drug information and knowledge from contract research organizations (which perform the bulk of pharma’s research) to publication planners (who direct the production of ghostwritten medical journal articles) to key opinion leaders (who are deployed to educate physicians about drugs) and beyond. In describing these paths I am describing circumstances of the production, circulation and consumption of medical knowledge; as a result, my project is about political economies of knowledge.
In pharma’s preferred world, research, education and marketing are fused. Our actual world is not so very far from that: when practicing physicians gain knowledge, they most often gain it from agents of big pharmaceutical companies, including local sales reps, researchers and educators sponsored to spread the word, and perhaps even journalists who needed a story to write. All these agents may aim to tell physicians the truth, but the truths they tell are drawn from streams of knowledge that have been fed, channelled and maintained by drug companies at every opportunity.
There is a ‘ghostly’ aspect to drug companies’ actions. My co-option of the ‘invisible hands’ metaphor draws attention to that, and ties ghostliness to pharmaceutical marketing. Research on markets has shown the extent to which they are created by institutions and constituted by concrete actions. In the case of pharma companies, much of the work to establish and exploit markets, the work to coordinate the production and circulation of knowledge, is performed by invisible hands.
Let me explore these themes a little further.
The Medical Knowledge Economy
We often hear the term ‘knowledge economy’ connected with pushes to increase countries’ high-tech spheres, scientific output and participation in higher education. Usually, the term refers to a ‘knowledge-based economy’, where the production of goods depends heavily on technical knowledge. In a knowledge-based economy, knowledge itself becomes an object of investment, trading, and deployment. In this book, I treat knowledge not merely as a productive resource, but as a good connected to other goods. I explore the circumstances of the production, circulation and consumption of pharmaceutical knowledge – what we might call the ‘political economy of pharmaceutical knowledge’.
Knowledge – and information more generally – doesn’t move on its own through its environments. For this reason, we can treat it as a kind of substance, rather than as something purely ethereal. In mundane terms, the knowledge we care about most is rarely easy to come by and spread. To create and establish valued knowledge typically takes resources, infrastructure, tools, skilled labour and considerable effort. A claim only becomes an established fact when it has been picked up by enough of the right actors, and woven into the fabrics of what they do and say. To spread facts isn’t much easier.1
I approach these topics from frameworks established in Science and Technology Studies. As a field, Science and Technology Studies does not provide a unified account of either the production or the distribution of knowledge, but it does always treat knowledge as something constructed, and not just waiting to be found.2 The field is based in the recognition that it is possible to regard the construction of scientific facts – through actions in laboratories and elsewhere – as the upshot of careful rhetorical work, and of work to establish what can be taken for granted: scientific knowledge is something produced.3 Science and Technology Studies also treats the movement of facts and would-be facts as something that could, in principle, be accounted for as the result of actions, rather than as a simple natural phenomenon.4
Because of this background, I am interested in how the pharmaceutical industry constructs and moves facts and claims, and this book will, I hope, provide some novel insights into that.
The quasi-substantiality of knowledge runs against the grain of some grand claims about new economies. Digital information can be easily reproduced and transmitted, and this makes it look as though new digital tools could create a very egalitarian political economy of knowledge, one in which the barriers to contributing to and accessing knowledge are modest. Wikipedia might serve as a good model of this egalitarian economy; it’s easy to edit and easy to read.5
Egalitarianism when it comes to knowledge is a laudable ideal, but the fact that knowledge is a form of cultural capital, already unevenly shared and constantly exploited to create new inequalities, makes it an ideal that is difficult to live up to. Actors come to knowledge arenas with differing amounts of cultural, social, symbolic and economic capital. This capital can be converted from one form to another, and the accumulation of capital depends upon the conversion not being transparent. Actors develop and deploy their capital to establish and change their relative statuses.6
Not only do medical researchers and physicians try to establish themselves as particularly knowledgeable, but the pharmaceutical industry helps them to do so. Pharma companies use their considerable economic capital to create and distribute other forms of capital: cultural, social and symbolic. Pharma companies ghost-manage the production of medical research, they shepherd the key opinion leaders (KOLs) who disseminate the research as both authors and speakers, and finally they orchestrate the delivery of continuing medical education (CME) courses. In so doing, they position themselves as the ultimate sources of the information physicians rely on to make rational decisions about patient care. In this we can see the importance of pharma’s hegemonies over medical knowledge.
Imprisoned by the Fascist government from 1926 to his death in 1937, the Italian philosopher Antonio Gramsci filled notebooks with thoughts about politics and culture. In his Prison Notebooks, Gramsci explores how a dominant actor doesn’t need to use overt coercion when it has hegemony over key institutions – as the Fascist government developed over the press, schools, religion and popular arts. In Gramsci’s thinking, hegemony establishes what is taken for granted or regarded as ‘common sense’ in different areas. In this book, I look at attempts to achieve hegemony over medical knowledge through contract research, publication in medical journals, the creation of medical culture via sponsorship of KOLs, and the continued dissemination of that culture via sales forces and patient advocacy organizations (PAOs). There are rough parallels between these institutions and those – like the press and schools – that Gramsci discussed.7
It is easy to talk about hegemony in hand-waving terms, finding some parallels or analogies between dominant interests and the actions or commitments of institutions – for example, between big business and elite newspapers. However, it is a challenge to identify and describe concretely the mechanisms that shape institutions and the views attached to them. Perhaps emblematic of the challenge, Gramsci writes that ‘Every social group … creates together with itself, organically, one or more strata of intellectuals, which give it homogeneity and an awareness of its function, not only in the economic but also in the social and political fields.’8
If Gramsci is right, the organic creation of intellectuals and ideas in dominant cultural groups tends to be hidden from view, naturalized within cultures. In the more defined sphere of medical knowledge, the mechanisms of cultural control are also at least partially hidden, and pharma companies’ roles have become naturalized. For many physicians, for example, pharmaceutical industry influence looks innocuous and ordinary, to the extent that they see the industry as the best source of medical information. The industry has achieved a level of hegemony over parts of medical education, and therefore over what physicians see as treatable diseases and how they should be treated.
However, the creation of intellectuals and the domination of institutions do not happen at all organically, but are instead the result of deliberate and careful actions. In this book, insiders describe in great detail how they and pharma companies aim for hegemony. They make their strategies and tactics visible to each other when they network and promote their services. They need to provide evidence to each other of the value of their tools and skills. Observers – like me – can eavesdrop by spending time at the perimeters of the industry.
This book is broadly about knowledge, but the issues at stake don’t fit well within traditional epistemology, the branch of philosophy that studies knowledge. Epistemology chiefly studies justification, and in particular tends to focus on the justification of beliefs as held by individual people. It is beyond doubt that some of the claims that drug companies make and promote are poorly justified, and some are false in egregious ways. On occasion, there are major scandals about errors, falsehoods and gross manipulations circulated by pharma companies – the ‘fake news’ of the medical world.9 But, by and large, these companies work within the medical mainstream, and produce data of reasonably high quality using the most valued of research tools; they go on to analyse it using standard statistical means, and construct articles that pass the scrutiny of peer reviewers at many of the best medical journals.10 The problems of knowledge in the drug industry discussed in this book are not primarily problems of justification.
However, seen in terms of political economies of knowledge, there are serious concerns about the practices of pharmaceutical companies. Largely unnoticed influence and control permeate important areas of medical knowledge. Individual companies with stakes in specific medical topics can influence knowledge so that their preferred science becomes dominant. The medical world then focuses on what the companies care about most, using the terms that they establish. Pharma companies can achieve hegemony over understandings of particular diseases, symptoms, treatment options, trajectories and side effects. Through the enormous resources at their disposal, they have staked out dominant positions on the overall terrain of medical knowledge. The drug industry has concentrated power to make particular medical knowledge salient, and the interests guiding that power are narrow.11
Very closely related to all of this are questions about agency, the capacity to act independently. In my account, I describe industry actors’ efforts to constrain and co-opt the agency of target physicians, patients and others. That is, pharmaceutical companies and their delegates try to persuade physicians and others to make decisions that align with the companies’ goals. Pharma’s efforts are successful enough that they invest in them over and over again.
The flood of knowledge that companies create and distribute is not designed for broad human benefit, but to increase profits. At least some of the time, broad human benefit and profits are in direct opposition to each other. Therefore, it is perhaps less pertinent to ask whether this or that piece of pharmaceutical knowledge is justified or true than to note instead that the structures that create and distribute pharmaceutical knowledge concentrate power in a limited number of entities with very narrow interests and defined goals.
The Invisible Hands of the Pharmaceutical Industry
Many otherworldly creatures occupy the dark spaces of human cultures. Ghosts, zombies, vampires and others – all not quite alive but feared for their attacks on the living – walk in shadows. Part of the mystique and terror surrounding these beings is rooted in the fact that we can’t quite see them, or can’t see them for what they are. Vampires, for example, can make themselves appear ordinary, or even, in some literary traditions, sophisticated, charming and aristocratic. They maintain their lifelike status by sucking the essence of life, usually in the form of blood, out of their victims.
Normally, the ‘invisible hand’ metaphor doesn’t carry any occult connotations. Adam Smith used it (very infrequently) to describe how individuals promote the interests of others or of society by acting in their own self-interest. The ‘invisible hand of the marketplace’ has come to stand for the processes by which innumerable real or possible selfish choices are thought to stabilize markets and optimize local utility. As groups, producers and consumers of a good should arrive at a price at which all of the good is sold and all of the demand is met. The invisible hand of the marketplace is, then, an effect of many visible hands.
In a classic book of business and economic history, Alfred Chandler describes how, in the nineteenth century in the US, there arose a new ‘visible hand’ of the marketplace, in the form of middle managers in new medium-sized and large companies. The planning carried out by these professional managers replaced some of the coordination previously effected by the free market, because it was more efficient and created more stability for the firms. On Chandler’s analysis, professional management allowed the largest of these companies to dominate sectors of the US economy and to reshape the larger markets of which they were a part.12
The pharmaceutical industry is immensely fond of invisible hands, but not Smith’s kind. The hands I make visible in this book are more like Chandler’s managerial hands. However, they try to maintain a ghostly status so that they cannot easily be seen, or cannot be seen for what they are. For pharmaceutical marketing to work best, it has to look like disinterested, unbiased, impartial medical knowledge. As a result, many of the hands doing the companies’ marketing work need either to be invisible or to look as though they’re doing something else. In this sense, Ghost-Managed Medicine is a study of the spectral in the pharmaceutical industry.13 The book follows spectral elements of first the production and then the distribution and consumption of medical information, along the path described just below.
The industry provides roughly half of all funding for clinical trials – often randomized, controlled trials (RCTs), the most valued form of medical knowledge – and sponsors most of the new trials initiated each year.14 The bulk of that funding goes to contract research organizations (CROs) and related firms. CROs plan and run clinical trials to get drugs approved and to make new cases for drugs to be prescribed. They recruit doctors, who recruit trial subjects, whose tissues, fluids and observable qualities can be transformed into data. CROs are the first of the phantoms in the drug industry, feeding on trial subjects’ bodies, but mostly staying out of sight in the medical research world. In the end, CROs make no claim on the data they produce; they simply turn it over to the companies that hire them to use as they want.
Using this and any other available data, the pharmaceutical industry produces a significant portion of the scientific literature on in-patent prescription drugs. In the more prestigious medical journals, as many as 40% of the articles on recently approved drugs have been ghost-managed for companies.15 I use the term ‘ghost management’ when drug companies and their agents control or shape multiple steps in the research, analysis, writing, publication and dissemination of science. Ghost management, I will show, is common. Some of the key ghosts are called publication planners, who design publication strategies, create teams of professionals to shape and write articles, select the journals they will be submitted to and choose KOLs to serve as the putative authors of these articles.
As a result of the work of CROs and publication planners, medical science is shaped to serve marketing goals. The drug companies’ interests can be expected to influence any number of choices in the design, implementation, analysis, description, and publication of clinical trials. We can reasonably expect – and there is abundant evidence – that the companies make choices to support their commercial interests. Even if companies are not completely coherent actors, they are coherent enough in their goals that choices at all the different stages of research and communication generally point in the same direction. The result is still recognizably medical science, and may even be high-quality science, but it is science designed to help sell drugs.
This continues with the communication of medical science in the field. When they give talks, KOLs contribute to the enormous influence that the drug industry has on medical knowledge. KOLs are the zombies of the industry, the animated bodies sent out to do pharma’s bidding – like the original zombies of Haitian folklore, who were created and controlled by sorcerers, and who served as slaves. Most KOLs are fully constrained; they present scripted presentations to other doctors and make the scientific and medical cases established by CROs and publication planners. The form of education in which KOLs participate is one thoroughly shaped by the companies that sponsor it. What KOLs communicate will often be sound medical science, and this is why they are willing to communicate it. Generally, they are fully committed to what they are doing; they believe their own talks and can easily justify their roles in marketing campaigns. The KOLs interviewed for this book defend giving promotional talks in idealistic terms: if physicians are ‘not educated enough, the public will suffer’, says one; ‘oh, it helps other patients elsewhere, it’s spreading the word – it’s spreading the gospel’, enthuses another. KOLs’ brains and souls have been taken over. They are sent out to take over other brains and souls, to convince more doctors of the evidence base for specific prescriptions.
There is a sophisticated service industry around all forms of medical communication. Marketers broadcast their ability to do ‘promotion through education’, claiming that CME courses can be ‘custom tailored to meet pharmaceutical marketers’ needs’.16 As agents of drug companies, medical education and communication companies (MECCs) create courses, plan conferences and seminars, conduct surveys and write articles and studies. All of this is then placed in the hands of the educators, researchers, and doctors who will use them to good effect. These firms feed stories to the journalists who write for newspapers and medical magazines, giving them technical details, journal articles, the names of experts to contact, and even narrative lines. They even provide video clips for television networks that then air stories about the latest advances.
Now for the boots on the ground: pharmaceutical sales representatives. These men and women work full-time to increase drug sales, which means convincing physicians to ‘change their prescribing patterns’. Variants of this phrase come up over and over again in pharma circles. The reps convince doctors to change their prescribing patterns by subtly boxing them in, effectively draining their agency, their ability to act independently. Sales reps arrive at physicians’ offices already knowing what their targets prescribe, how they see themselves, and a host of other small facts that might help to establish rapport. They are also armed with sets of scripts for most occasions, so they are prepared for doctors’ evasive moves. The result is that even if doctors see themselves as making their own decisions through the interactions, the sales reps are well placed to make those decisions lead to fresh prescriptions of the drug under discussion. Doctors feel that they are in control of the situation and their actions, while sales reps are stealthily ‘changing prescribing patterns’. These sales reps make good use of the scientific studies that drug companies commission and shape. Medical science sells drugs by allowing doctors to make justifiable decisions.
Patient advocates and patient advocacy organizations are further important nodes in the shadowy marketing of drugs. Two-thirds of PAOs in the US receive industry funding, and the organizations within that group receive 45% of their funding from pharmaceutical, medical device and biotechnical companies.17 Some 93% of PAOs that make presentations before or participate in discussions within the US Food and Drug Administration (FDA) receive industry funding.18 The situation is similar in other high-income countries such as the UK.
In extreme cases, PAOs are creatures of the industry. They are fully funded by one or more companies, staffed by professionals, and find patients to be members after the fact. They, like many other funded PAOs, serve as lobbyists and do public relations work, promoting drugs and diseases and defending pharma against negative publicity. They are sirens for the industry, singing passionately about better futures with better drugs. And as for pharma’s other phantoms, invisible hands are busy manipulating other actors, working diligently to disguise motives and interests.
Overall, then, pharma companies rely on systematic pressure on the circulation of scientific knowledge and the resulting medical practices. This is a system of influence made more effective by being shadowy and spectral.
Because I focus on ghostly marketing within and immediately around medicine, I won’t address more overt kinds of marketing in this book. For example, in 2016 in the US – the large country most open to drug ads – pharma as a whole purchased more than $3 billion in television advertising, and spent nearly as much on ads in magazines, newspapers and other media. Of that, $300 million went to ads in medical journals.19 It may be that ad spending gives companies some leverage over television networks and other media, including medical journals, and thereby expands pharma’s influence. However, to limit the areas I address, I don’t explore that leverage here.20
Pharma companies have many agents over which they have direct control, such as the companies, firms, agencies and consultants they hire for specific purposes or to create specific products. By outsourcing to these agents, the companies take advantage of external expertise and resources, and extend their reach. The agents I describe in this book, which include the ones on which companies spend the most money, are hired to produce or transmit information to be taken up by other elements of the market, including regulators, physicians and patients. The companies and their agents influence those other elements by shaping what they know and believe. Regulators, physicians and patients then act in ways that seem rational, obvious or easy. To the extent that the companies are successful, they constrain the agencies of their targets in much the same way that an expert chess player can constrain the agency of a more novice opponent across the table.
An Expansive View of Marketing
Total drug sales rise nearly 10% per year, through good times and bad.21 This suggests that we still need some large stories about the efficacy of marketing and the demand for drugs.
The ‘market’ of neo-classical economics is a metaphor. Markets were once physical spaces, where sellers and buyers would meet to exchange goods and money. Of course, all sorts of other things happen in traditional markets besides buying and selling: carting goods in, setting up stalls, socializing, theft, and pretty much anything else that happens when people gather. But the original metaphor has almost always been used with a narrow focus on planned buying and selling.
In the tightly packed physical market that is the source of the economists’ metaphor, goods of the same kind sold by different vendors quickly end up fetching the same price, because anybody charging too much is unable to compete with vendors in the next aisle, and anybody charging too little realizes that they could make more money by closing the gap between themselves and their competitors in the stand across the way. Profits should tend to drop, because whenever there are opportunities for high profits on one kind of good, multiple sellers should switch to making and selling more of that good, bringing the price down. Overall, the logic of the metaphor points toward an efficient price mechanism that balances supply and demand. Today, actual markets are mostly regulated to make them behave like the markets of economists’ metaphor.
The modern corporation, company or firm is an institution for evading the market of neo-classical economics. Obviously, firms have no interest in seeing their profits drop to zero, so they are engaged in continuous wars on free markets. Activities such as branding and advertising attempt to make products incommensurable, to establish monopoly control and to increase the number of buyers and the price for a particular product. For example, to the extent that different strains of rice are broadly comparable, that new sellers can enter the rice market, and that there isn’t a tremendous imbalance of resources among buyers, eventually rice prices should tend toward a level equal to marginal costs and profits should drop. Enter Nishiki brand ‘New Variety’ rice, advertised as superior to other rice brands and of a consistently high quality. If consumers agree that Nishiki is not strictly comparable to other rice, then the Nishiki company will be able to maintain profits.
Firms arise and evolve to avoid free markets, using a variety of strategies more available to corporate bodies than to individuals – such as having managers dictate employees’ actions, thereby reducing ‘transaction costs’.22 In this book, I emphasize one set of reasons based on firms’ abilities to marshal resources to shape or control markets for their own benefit.23
While some people might be temperamentally comfortable living with uncertainty, firms engage in careful planning to limit the effects of uncertainty at every turn. Economist John Kenneth Galbraith identified a number of strategies that modern corporations use to deal with uncertainties in supply and demand. In the extreme case, they can simply take control over uncertainty. Galbraith writes: ‘This consists in reducing or eliminating the independence of action of those to whom the planning unit sells or from whom it buys.’24 In other words, firms depend upon the coordination and delegation of both inside and outside actions, rather than the rational actions of independent actors.
Pharmaceutical companies would control all the actions of market gatekeepers and customers if they could, but they can’t. Instead, they do the next best thing and engage in campaigns of influence, subtly reducing the independence of the actors they need to sell their products. Because of a number of unusual features of the drug business, this can be a very successful strategy.
We might see pharma companies as engaging in ‘channel marketing’, influencing various ‘channel partners’ in order to access customers.25 A company controls or influences channel partners, which then influence either customers or still more channel partners. With strong enough bonds, the company will eventually control the whole channel between itself and the customers. Figure 1.1 provides a highly schematic image of the interactions involved.
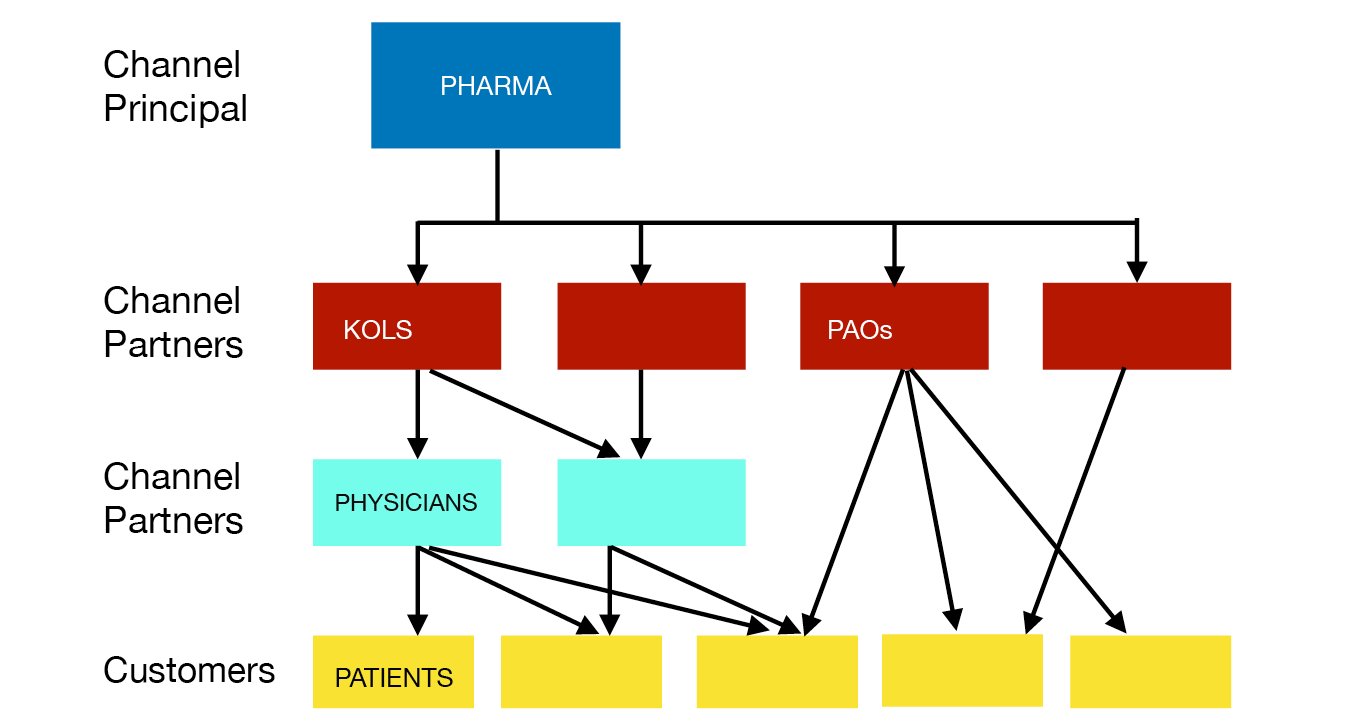
Fig. 1.1 Channel marketing
One unusual feature of the drug business is that the most profitable products are available only by prescription. As a result, pharmaceutical companies focus their marketing attention much more on prescribers than on end consumers. They want physicians to make favourable diagnoses, and then to write prescriptions for their products. Health and medicine are forbiddingly complicated subjects, and drugs are dangerous. Consumers – that is, patients – typically lack the knowledge to be considered competent stewards of their own treatment, and also lack the knowledge to evaluate or challenge their doctors’ assessments and recommendations. Patients have limited choices when it comes to many drugs; usually they are allowed to decide only whether to fill the prescription and whether to take the drug.
Health, medicine and drugs are such complicated subjects that even many physicians lack the knowledge to independently evaluate or challenge claims that appear in medical journals or clinical practice guidelines. Many doctors are in the position of having to choose primarily whom to trust, rather than what to trust.26
In addition, end consumers of the most profitable drugs often don’t fully pay for them. In wealthy and middle-income countries, different kinds of public and private insurers provide the bulk of the funding for expensive treatments. These insurers are gatekeepers of a different kind than are physicians, and so become another marketing target for pharma companies. They are increasingly important targets, because healthcare costs keep increasing and insurers appear to be trying to contain their spending – including on drugs.
In its everyday use, ‘marketing’ is a term for actions to promote products, perhaps by advertising and branding. The American Marketing Association has a much broader concept of marketing, defining it as ‘the activity, set of institutions, and processes for creating, communicating, delivering, and exchanging offerings that have value for customers, clients, partners, and society at large’. In this definition, not only have the traditional physical markets of the original market metaphor disappeared, but so has the narrow focus on planned buying and selling.27 The modern world is leaving the market of neo-classical economics behind.
In the ‘marketing era’ captured by this expansive definition, products (or services) don’t simply arrive at a marketplace to be sold. Companies don’t merely try to satisfy pre-existing needs, but identify opportunities to shape needs and the means of satisfying them.28 In the ideal case, every step in the trajectory of manufacture, advertisement, transportation, sale, delivery and consumption will have been shaped by every other step. Products should be designed with their future paths in mind, and consumers should be created with products’ paths to them in mind. Products, pathways and consumers should all be shaped so that they meet in pre-arranged harmony.
In the context of the pharmaceutical industry, the American Marketing Association’s definition would include anything that drug companies – or any body or person used by the companies – do to get their products into consumers’ bodies.29
I find the American Marketing Association’s definition of marketing useful, because the activities pharmaceutical companies engage in to create sales are not neatly bounded. Take clinical trials, for example. Early clinical trial work simultaneously identifies good candidates for drugs and defines potential markets – most of the time, these tasks are identical. A clinical trial can serve to convince regulators to allow a product onto the market, and to allow it to be advertised as useful for certain medical conditions; without that permission there will be no sales. A trial can serve to provide evidence that will help convince doctors to prescribe drugs and insurers to pay for them. A trial can help create a buzz around a product, through reports on it placed in medical journals and the popular media. A trial can suggest new, unapproved uses for a drug. A trial can put a drug in the hands of physician-investigators, who will then prescribe it more frequently. A trial can establish relationships with investigators, who can later be called upon to speak on behalf of the studied drug and others. A trial can enrol patients, who may continue to use the studied drug after the trial is over. An ongoing or future trial can serve to delay answers to questions about a drug. Every single one of these uses of trials contributes to marketing drugs.
Marketing in the marketing era is still essentially a set of intentional activities. It is done by companies and their agents, but not by independent actors, who merely happen to increase sales. My focus on marketing in this book is also a focus on some of the marshalled forces at work.
Pharmaceutical companies outsource a great many of their tasks. The vast majority of clinical research, one of the companies’ largest costs, is outsourced to CROs and private site management organizations, and to a lesser extent to academic researchers and the academic research organizations that universities have set up to compete with CROs.
Although companies still do some of their own drug discovery and development work, an increasing number of the drugs in their pipelines are first developed by biotechnology companies and startups of one kind or another. They then move into larger companies through licensing arrangements or the acquisition of the smaller companies by the bigger ones. Small companies just don’t have the capacity to market anything other than the most specialized drugs.
Pharma companies also outsource to medical education and communication companies (MECCs) much of the development of publication plans, the writing of medical science articles, other articles, promotional presentations, medical education programmes, and more. The companies even outsource parts of their marketing planning, and may make agreements with other companies to hire or share sales forces for particular projects.
So, what is a pharmaceutical company, if so much of its work is done by outsiders? The companies keep core competences in all of their functions, so that they can intelligently manage all of their projects. Some of them have internal strengths – for example, some maintain expertise in the development and production of vaccines. But most importantly, the companies engage in high-level planning, both long- and short-term. They create, stake out and defend positions.
A company engages in marketing by pushing different agents, groups and entities together to create a unit that works well and is much stronger and more powerful as a result. I call this ‘assemblage marketing’. The ideal result is a market that not only buys the company’s drugs, but is permeated through and through by acceptance of and interest in those products. It is a market designed so that to purchase particular drugs is rational, or the path of least resistance.30
Figure 1.2 sums up the central narrative of this book. Through various agents, a pharmaceutical company creates a market by producing, shaping and transporting research and medical journal articles, as well as key opinion leaders and patient advocates. It pushes these in the expectation of influencing regulators, physicians, patients and other useful actors.
In this picture, markets are made, not born.31 The idea of assemblage marketing suggests that a market can be created for any product, given enough resources. This point suggests that the less obvious the initial assemblage of elements for the eventual market, the more resources a company must put into shaping and moving those elements. Eventual demand is a product of initial demand and marketing effort.
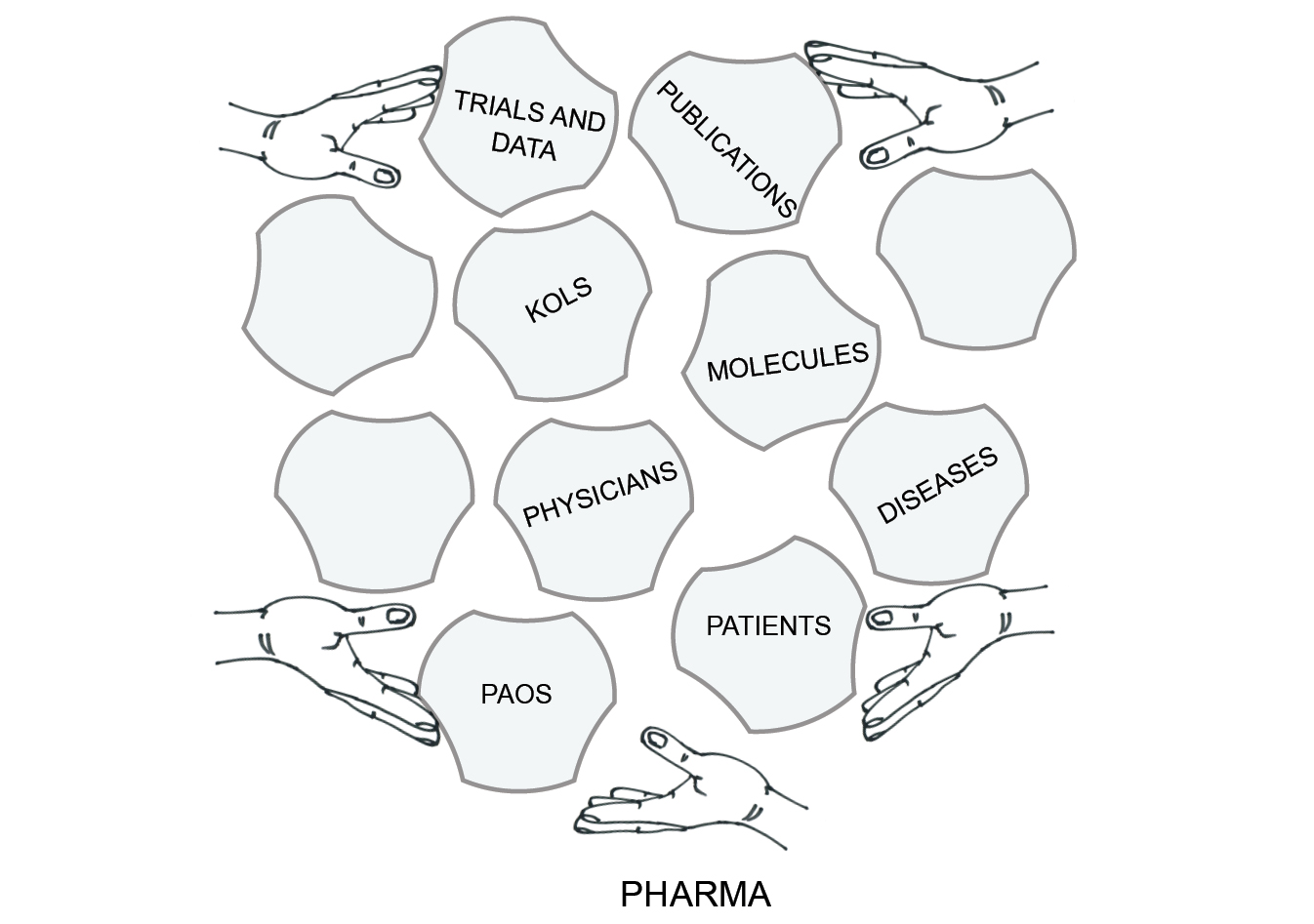
Fig. 1.2 Assemblage marketing
For the company, the result of assemblage marketing is much more than the sum of its parts, because – to the extent that it is successful – the assemblage is constructed with the company’s interests in mind, these interests being expressed through its preferred medical knowledge, assumptions and practices.
Shaping Patients and Diseases
Tucked into the bottom-right of my diagram of assemblage marketing is a tile labelled ‘diseases’. Like the elements on all of the other tiles, diseases can be shaped and adjusted to make a stronger and more profitable market.
In the 1960s, critics railed against the authority of physicians, who were seen as medicalizing intimate events and processes. Criticism of medicalization was especially prominent in challenges to psychiatry by scholars such as Michel Foucault and Thomas Szasz. Their work was later picked up and applied in different realms, especially by feminist scholars. As a result, there have been challenges to, in particular, mental health diagnostic categories ranging from schizophrenia to anxiety and depression, and also to the medicalization of ordinary life events and stages, such as childbirth and menopause.
The focus on professions has more recently lost some of its traction, and physicians today look like only one set of actors in the struggle for control over bodies, health and illness. Today, medicalization looks as though it is merely grease for the wheels of pharmaceuticalization – the objects of most critics’ attention, for example anxiety, depression, and menopause, are closely associated with new classes of drugs.32
To increase their sales, pharma companies try to ‘sell sickness’. They work to expand awareness of diseases for which their drugs can be prescribed, and to increase the likelihood that people will see themselves as having those diseases.33 Sometimes this work is subtle and long-term, accomplished by building a corpus of medical science on a condition and systematically promoting disease labels and products. In other cases, however, it is more focused, as when pharma companies create defined ‘disease awareness campaigns’ to have an immediate impact.
An announcement for one conference on disease awareness campaigns states that it is intended ‘for representatives from pharmaceutical, biotechnology, and medical device companies and advocacy groups with responsibilities in the following areas: disease area marketing, patient experience, patient engagement, integrated marketing, health system engagement, public relations, digital marketing, communications, multichannel marketing, content strategy, patient strategy, product development commercial strategy’ and so on.34 Almost all of the responsibilities listed appear to be aspects of marketing and communications, and it isn’t obvious that the medical affairs side of pharmaceutical companies is represented anywhere on the list.
Depression is one of the most obvious and important diseases affected by the availability of drugs. Until the 1960s, depression was a relatively uncommon diagnosis, and tended to be associated with the elderly.35 It became slightly more prominent in the 1970s, promoted by makers of the first generation of antidepressants.36 Since the arrival on the market of Eli Lilly’s drug Prozac in 1987, however, ever-increasing numbers of people have been diagnosed with depression. The number of people disabled by depression has increased, diagnostic criteria for depression have continually broadened, and estimates of the prevalence of depression have gone up dramatically.37 It is now the ‘common cold’ of mental disorders.
Prozac, or fluoxetine, was the first successful drug within the Selective Serotonin Reuptake Inhibitor (SSRI) family, now often called simply ‘antidepressants’. Companies selling SSRIs have marketed both the drug and the disease. They have invested heavily in research on depression and antidepressants. They have widely promoted a serotonin-deficiency theory of depression, followed by a chemical imbalance theory, for neither of which there is much evidence. They have established close connections with psychiatrists and other physicians who write textbooks, articles, and clinical practice guidelines. They have sponsored awareness and anti-stigma campaigns. The companies have successfully established the disease both medically and culturally, helping physicians to recognize and diagnose it often, and helping patients to interpret their feelings and experiences in terms of it – perhaps even to shape their identities around it. The World Health Organization predicts that within twenty years more people will be afflicted with depression than with any other health problem.
Depression may seem like a special case, both because it is a mental illness and because the boundaries between the disorder and sadness are imprecisely defined. However, there are many examples of more ‘bodily’ illnesses that have been strongly affected by marketing efforts, including common chronic diseases such as hypertension, diabetes, high cholesterol and osteoporosis.38
To take one of these examples, when the company then known as Merck Sharp & Dohme introduced an anti-hypertensive drug, Diuril (chlorothiazide) in 1957, hypertension (high blood pressure) was a sign associated with underlying poor health. At that time, high blood pressure wasn’t itself generally seen as a problem, and therefore wasn’t something to be controlled. Even defining high blood pressure was difficult, since blood pressure was represented within populations using a bell-shaped curve, so any dividing line between high and normal was artificial. Diuril dramatically lowered blood pressure, apparently only in people with elevated levels, suggesting that it might address some root problem. Together with an amazingly successful marketing campaign, its effects established hypertension as a disease.39 The mild side effects of Diuril and other diuretic drugs made it straightforward for companies and their agents to argue for diuretic use to treat ever-broader populations with ever-lower blood pressures. Recommendations followed suit, and blood pressures that were once judged as within a normal range became perceived as high. People came to understand themselves as hypertensives or at least as disposed to hypertension; in one case, that of hypertensive African-Americans, the condition has become linked to racial identity.40 The pharmaceuticalization process has continued as new antihypertensive drugs have been found.
Chronic conditions like hypertension neatly fit a new model of health and illness. Once upon a time, people generally considered themselves healthy unless they felt ill, or had unusual frailties or symptoms. But two major changes have resulted in a new model of health. First, in the past half-century we have seen the rise of risk factors: familiar things such as diet, age and sleep patterns, and unseen and unfamiliar things such as cholesterol levels, positive BRCA1 and 2 genetic tests, and PSA (prostate specific antigen) readings. We are all at risk, differing only in degrees. Second, and partly as a result of the first change, we can be normal and unhealthy at the same time, at least when there is some hope for treatment. For example, the unfortunate results of ageing used to be just that, but now we look for medical means to stave them off or treat them. As a result, there is no contradiction in the thought that most of us are less than healthy in this or that respect. In addition, there is no limit to the potential demand for health. We are all always unhealthy. Most of the ways in which we are unhealthy are chronic, so treatments can extend for life. And since we are all unhealthy in so many ways, treatment – even successful – of risk factors or conditions allows us to focus on new problems.41
Drugs don’t define diseases by themselves. But drugs affect diseases, as do pharmaceutical companies through disease awareness campaigns. The companies change categories and the material ecologies of diseases, and in the process sometimes make people diseased who weren’t before.
This Book’s Sources
The pharmaceutical industry is secretive about many things. It is extremely difficult to get inside drug companies, and especially difficult to get information about the research and marketing of particular current and recent drugs. Researchers who have managed that task have often relied heavily on documents becoming publicly available through lawsuits.42 Where it makes sense to do so, I will draw on such documents. But there are also other ways to dig deeper into the industry and its practices.
As I’ve mentioned, drug companies outsource many activities to outside agencies. This creates a need to communicate. The agencies need to promote and advertise their services. People in the agencies communicate amongst themselves and with drug companies about the services they offer, the tools they use, and best practices. And all the people involved, both inside and outside the drug companies, need to network. As a result, there are newsletters, workshops and conferences focused on different aspects of pharma. I think of these as penumbral, sitting in an imperfect shadow of the industry. In these partial shadows, we can see some of the industry’s phantoms talking about and displaying what they do.
Writers in these publications and speakers at these events describe the practices of the drug companies and agencies they are allied to. They often speak candidly about the goals they are expected to meet, the problems they face, and the solutions they’ve found, though they almost always disguise the products in question. From their accounts, I have gained a picture of how drug companies attempt to construct, shape and spread medical knowledge. I have to be cautious about what these writers and speakers claim, because they are always promoting themselves, and are apt to exaggerate their influence.43 However, they often provide case studies with revealing details, give evidence of their effectiveness, and corroborate each other’s accounts. As a result, it’s possible to form a reliable impression of drug company work.
To put together this book, I attended thirteen of these penumbral meetings, listening to several hundred presentations on a variety of topics; my research associates attended four more such meetings. Three of the meetings took place in Europe (in Berlin, London and Vienna) and the rest in the US (in cities from New York and Philadelphia to San Diego). As a result, I have more US examples than European ones, though I have tried to reduce the imbalance in this book by selecting good European examples wherever appropriate. Most of the structures I discuss are international in nature and apply similarly to most wealthy countries, though on some issues there are important local legal and regulatory differences. I do, however, start with one long and (so far) peculiarly North American case, a story of the marketing of the painkiller OxyContin. This case is derived from the secondary literature, rather than my primary sources, but it illustrates the processes and the stakes of pharma’s marketing efforts.
As much as possible, I tried to be a fly on the wall at pharma conferences. Even wearing a badge with my name and university affiliation, this was easier than it might sound. At the coffee and pastry tables, at lunches and receptions, people would ask some version of ‘What is an academic like you doing here?’ I would reply by saying something about my field of Science and Technology Studies, and how it studies knowledge production and knowledge management. Before I had finished a few sentences, most interlocutors’ eyes were glazing over: they were at the conference to network, and I was going to be of absolutely no value to them.
Meetings are useful events, where you can learn a lot about the structure of work. At the meetings that my colleagues and I attended, speakers talked about goals, problems, conflicts and organizational structures.44 I recount only things that were said in public, mostly from the podium, though occasionally in a question from the floor. I don’t report on catty hallway conversations, but rather present the part of the pharmaceutical industry that is public – albeit within its own shadows.
Because these were public meetings, it is difficult to fully ensure the anonymity of speakers. Nonetheless, I don’t refer to them by their own names. Moreover, I take the views of almost all of these speakers to be representative of their colleagues more generally, so there is no need to highlight their identities. I give them pseudonyms that reflect their work. For example, I assign people working in the medical side of pharma – in medical affairs departments or as medical science liaisons – common names that begin with M, such as Mr Moore or Ms Morales (see Fig. 1.3). I try to reflect very roughly the range of ethnic origins of actual names in my pseudonyms.
In addition, I draw on anonymized interviews with a small number of industry actors and with more than a dozen physicians and researchers who have worked as speakers for drug companies. For additional material and to corroborate what I learned elsewhere, I have read widely in the parallel newsletters and magazines – the ‘grey’ literature of the industry. When I quote from printed sources rather than meetings, I attribute words to their actual authors, either in the text or the endnotes; given the citations, anonymity is not possible.
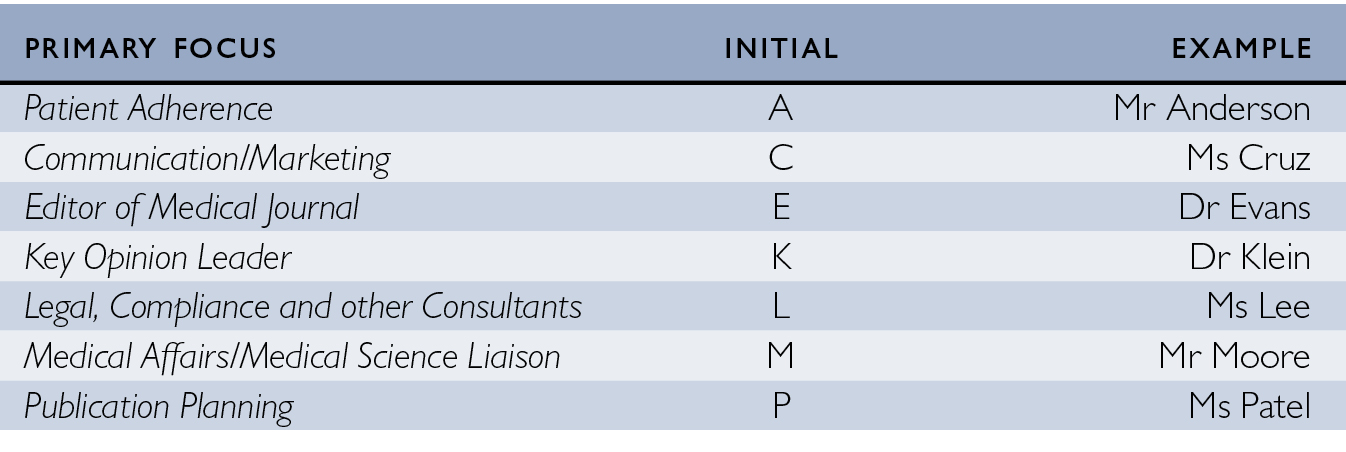
Fig. 1.3 Pseudonym system used in this book
In most of the chapters of this book, I give space to these many speakers, the spectral hands of the industry. They often speak in idealized terms, outlining goals and the tools with which they hope to achieve them. When they speak about achievements, they tend to focus on successful cases, rather than fully successful campaigns. As a result, this book presents pharma’s aspirations and strategies, and does not portray a fully achieved hegemony. Nonetheless, especially when we put the different phantoms’ accounts together, what they present is chilling.
Coda: An American Nightmare
In the rest of this chapter, before turning to the different agents who shape and move information for the pharmaceutical industry, I present a cautionary tale. It is an extended example that includes almost all of the elements of the rest of this book. For the most part, it is a very ordinary tale of drug marketing, except for the inherent dangers of the particular drugs and the scale of the disaster they created.
The number of drug overdose deaths in the US and Canada has been increasing by approximately 20% per year since the year 2000.45 In that period, more than 200,000 North Americans have died from prescription opioids.46 Prescription opioid abuse has cost the affected economies billions of dollars.47 The situation is only getting worse, because in recent years users have increasingly been forgoing prescription opioids for heroin and, latterly, illicit fentanyl and carfentanyl – the last two of which are extremely powerful painkillers now often mixed in with other street drugs to increase potency.
How did millions of North Americans become addicted to opioids, leading not just to deaths but also to huge numbers of devastated lives and much pain for the family members and friends of those addicted? The story is complicated, and has elements that range from the US invasion of Afghanistan in 2001 to the anomie felt by too many North Americans. But a central part of the story is particularly important for this book: the pushing of prescription painkillers, especially OxyContin. My short account here makes connections with most of the themes of this book: the marketing of OxyContin was in most ways exactly the same as the marketing of any other major drug. However, the story of OxyContin is different, because of the devastation that followed.48
The US Food and Drug Administration (FDA) approved OxyContin for sale in 1995. The painkiller in OxyContin is oxycodone, an old – invented in 1916 – morphine derivative, similar to heroin in its structure and overall effects. The selling point of the new drug was high concentrations of oxycodone in each pill combined with a continuous-release mechanism, which its maker, Purdue Pharmaceuticals, called ‘Contin’. The Contin mechanism, which had been patented in 1980, was supposed to moderate euphoric and similar effects, and provide pain relief for twelve hours. The result, it was claimed, was a minimally addictive opioid. For Purdue, OxyContin was a good replacement for its previous slow-release opioid, MS Contin, which had a record of being abused as a recreational drug.
The FDA set the rules for the marketing of OxyContin, making it possible to produce, promote and distribute the drug. The initial ‘label’ for OxyContin approved it for the treatment of pain associated with musculoskeletal conditions, and later expanded it to include other conditions. At any time after the drug’s approval, doctors could prescribe it for whatever conditions they saw fit, but Purdue could not promote it for anything beyond the label. As it happened, Purdue exceeded its mandate, but stayed close enough that it was not caught for a few years. Curiously, the initial label noted that, when properly prescribed, addiction was ‘very rare’,49 a claim that was quickly challenged. But the FDA had established the framework for the promotion of OxyContin. Meanwhile, the US Drug Enforcement Agency, which authorizes production quotas of potentially addictive painkillers, allowed the production of oxycodone to increase nearly forty-fold from the early 1990s to today.50 There has been a twenty-fold increase in the opioid family of drugs during this time. Purdue made huge profits, and the Sackler family that owns the company became enormously wealthy; the Sacklers are known for their philanthropy, making highly visible donations to art museums and universities.51
Early promotion of OxyContin, often known on the street as ‘Oxy’, involved recruiting doctors, pharmacists and nurses to the cause of aggressive pain treatment. Purdue’s Oxy marketing plans from 1996 to 2001 included inviting more than 5000 attendees to over forty lavish, all-expenses paid conferences in pain management and speaker training.52 These conferences established a prescriber base for Oxy, and more importantly, a base of key opinion leaders (KOLs) to sit on Purdue’s speaker bureau, to give paid presentations to other prescribers. The company’s speaker bureau list included 2500 doctors, of whom 1000 were active.53 With this KOL force, Purdue sponsored more than 20,000 educational events to make the case for using opioids to treat pain aggressively.
Purdue made an arrangement with the Joint Commission on Accreditation of Healthcare Organizations, which had issued pain standards for hospitals. By sponsoring the work of the Joint Commission (an ‘independent, not-for-profit organization’54), Purdue gained the exclusive right to distribute educational materials, which gave the company access to hospitals seeking accreditation.55 In one analysis, the Joint Commission’s new standards for pain management in the year 2000 was one of the two most important events driving the opioid epidemic, the other being the introduction of Oxy itself.56
Purdue didn’t have a large enough sales force to market OxyContin, so it established an agreement with Abbott, a much larger drug company with a broad array of products. Abbott had the foresight to include in its initial agreement a clause stating that the company would have no legal responsibility for the drug, making Purdue the drug’s sole legal and public face. (Still, Purdue’s legal costs so far have been relatively small, in light of the effects of the drug and the profits that the company has raked in.)
Both Abbott and Purdue worked their Oxy sales force hard. The average bonus for Purdue sales reps in 2001 was $71,500, an amount considerably higher than their $55,000 average salary. Abbott offered cash prizes and luxury vacations to top sellers. Meanwhile, sales reps were coached on how to woo doctors with food, how to connive their way to getting three or five minutes of doctors’ time to make pitches, and how to position the product. Digging through internal Abbott documents, David Armstrong reports ‘an almost religious zeal’ to sell the drug:
Sales reps were called ‘royal crusaders’ and ‘knights’ in internal documents, and they were supervised by the ‘Royal Court of OxyContin’ – executives referred to in memos as the ‘Wizard of OxyContin’, ‘Supreme Sovereign of Pain Management’, and the ‘Empress of Analgesia’. The head of pain care sales, Jerry Eichhorn, was the ‘King of Pain’ and signed memos simply as ‘King’.
‘As you continue to carry the OxyContin banner onto the field of battle, it’s important to keep highlighting OxyContin benefits to your doctors’, Abbott urged its sales staff in a memo contained in the court records.57
In addition to free samples left at doctors’ offices, in 1998 Purdue created a patient starter coupon programme for OxyContin, to provide a number of patients with free initial prescriptions of between seven and thirty days. As part of the programme, doctors were given coupons they could pass on, thereby helping disadvantaged patients. Purdue exhibited all the generosity of a neighbourhood drug dealer with a potential new customer.
In the years following the introduction of OxyContin, a number of medical journal articles made the case that very few opioid prescriptions led to addictions. Strongly suggestive of a promotional campaign in the medical literature is the fact that a 1980 letter to the editor of the New England Journal of Medicine that claimed that fewer than 1% of hospitalized patients treated with opioids became addicted was cited more than 600 times, with citations spiking after 1995. Most letters to the editor are lucky to be cited even one-tenth as often.58
Purdue and other drug companies have intervened more broadly in the medical science of opioids. As of 2017, the nine most cited reports of randomized controlled trials of oxycodone in medical journals were all funded by Purdue or one of the network of international companies spun off by Purdue. A majority of the many authors on these articles – most have six or more authors – are apparently independent medical researchers, though corporate authors are sprinkled around. None of these nine influential OxyContin trial articles describes in any detail who conducted the research, who did the statistical analysis, who wrote the article, or who did the shepherding necessary to submit it to a journal, make the needed revisions, etc. In other words, these reports were almost certainly ghost-managed. These are only the most cited reports of randomized controlled trials; many more articles, including reviews, commentaries and less cited reports, may also have been ghost-managed for Purdue.
One of these influential medical journal articles failed to report some cases of withdrawal symptoms, cases that could be argued away.59 Whereas the article reported withdrawal symptoms in only one of 106 patients, an internal review found that eleven others reported negative experiences on the drug, these being at least possibly due to withdrawal symptoms. An ‘agreed statement of facts’ from a legal action included an account of the following episode:
[A] PURDUE employee emailed a PURDUE supervisor regarding the review of withdrawal data …: ‘Do you think the withdrawal data from the [osteoarthritis] study … is worth writing up [an abstract]? Or would this add to the current negative press and should be deferred?’ The supervisor responded: ‘I would not write it up at this point’.60
The journal article was reprinted 10,000 times, to be given to doctors.
There is a widespread belief (vigorously denied by Purdue) that the pills don’t provide pain relief towards the end of their twelve-hour dosing period, creating a ‘cycle of pain and euphoria that fosters addiction’.61 In the face of this and other increasing concerns about addiction, the industry latched onto a convenient and somewhat speculative concept: pseudoaddiction. Pseudoaddiction is not a genuine addiction, but is instead a condition in which patients receive inadequate doses of opioids to manage pain. These patients display the signs of addiction, but only because they are suffering from their underlying pain. Therefore, runs the inescapable logic, rather than attempting to wean patients off opioids, the medical community should prescribe them more! Purdue has indeed recommended responding to inadequate treatment with bigger doses, though critics suggest that in the context of the twelve-hour problem bigger doses create ‘higher highs [and] lower lows’.62
There is little evidence for the existence of pseudoaddiction. However, lack of evidence has not stopped a great many medical articles from using the concept in an uncritical way, especially review articles, clinical guidelines and commentaries.63 We cannot know how many of these were strongly influenced by pharma companies. However, a small number do acknowledge pharma support: nine of those twenty-two acknowledge support from … yes, Purdue.64
Purdue and other companies producing opioids have also contributed generously to education about pain – producing a book often given away to medical students65 – and to organizations such as the American Geriatrics Society and the American Academy of Pain Medicine. On an American Geriatrics Society panel that wrote guidelines for the treatment of chronic pain in seniors, more than half of the members had been paid for consulting or speaking by one or another of the companies that manufacture opioids.66 More recently, organizations that have received funding from manufacturers of opioids have tended to oppose precautions about prescribing.67
The promotional efforts were extremely successful in some areas. For example, between 2007 and 2012, more than 200 million doses of Oxy were shipped to pharmacies in West Virginia, a sum that amounts to more than 100 pills for every adult and child in the state. Purdue had found its markets, based on presumed epidemics of untreated pain. During that period, there was a steady increase in sales of the higher-strength pills, consistent with a growing rate of addiction.68 Drug distributors, which knew exactly to which towns and pharmacies the pills were being sent, didn’t raise the alarm, even when they were legally required to; they made billions of dollars in profits.69
In 2007, three of Purdue’s executives were convicted on criminal charges for misleading doctors, and the company paid $600 million in fines.70 There have been a great many more lawsuits since then, some of which have involved settlements, and some of which are ongoing. However, the total amounts that the company will pay out in fines and settlements are trivial compared with the amounts it has earned.
OxyContin’s sales were disproportionately rural. Taken recreationally, Oxy became known as ‘hillbilly heroin’. Why was Oxy so strongly associated with places such as Kentucky, West Virginia, Ohio and Maine, or in Canada, with rural Ontario and Newfoundland?
Purdue, followed closely by its competitors, had put much more effort into promotion and sales in rural than in urban areas.71 Purdue consistently targeted those doctors who were the highest prescribers of opioids; this skewed the company’s marketing toward areas with a history of opioid use, such as the Appalachian area, and in general toward rural areas with older populations. Although the eventual users of OxyContin cut across generations, older people dealing with pain were the first market.
Drugs always have cultural aspects, and these are especially obvious for illegal drugs.72 As the use of OxyContin spread, it became part of the fabric of a number of local cultures, perhaps including cultures of sharing medications, and certainly as a way of dealing with social and economic problems. Oxy flourished where it first became common. The original safety warning on OxyContin advised patients not to tamper with the pills:
Warning: OxyContin Tablets are to be swallowed whole, and are not to be broken, chewed, or crushed. Taking broken, chewed, or crushed OxyContin Tablets could lead to the rapid release and absorption of a potentially toxic dose of oxycodone.73
As observed in a 2004 US General Accounting Office report on problems with OxyContin, this label may have ‘inadvertently alerted abusers to possible methods for misuse’.
Finally, there were larger issues of distribution. Once Oxy and other prescription opioids became common street drugs, they had to compete with other street drugs. Most pharmaceuticals only have to compete with each other, with alternative treatments, and with other approaches to health. In this case, however, prescription opiates were going head-to-head with heroin and other drugs that produce euphoria.
Oxy and its kin had a built-in advantage, though. The main distribution system for prescription drugs is usually entirely legal, going from manufacturers to wholesalers to pharmacies to patients with prescriptions. The drugs only become illegal if pills are stolen, the prescriptions are fake, or patients sell or give pills to other users. Drugs like heroin, on the other hand, are illegal at every step of the distribution system. With heroin arriving in North America at major cities and ports, the first points of its distribution are in those high-density centres. Through the first decade of the twenty- first century, the price of heroin and Oxy was similar enough that distribution systems made the difference: heroin sold better in major cities and on the coasts, and Oxy sold better in rural areas and the interior.
One of the most effective forms of distribution of Oxy was through ‘pill mills’. Doctors would set up offices, often in the form of stand-alone pain management clinics. They would see patients for a minute or two, prescribe a month’s worth of high doses of painkillers and other popular drugs, and collect a fee in cash. The most successful pill mills dispensed the drugs through their own pharmacies, making a profit on both the prescription and the drugs. There was so much cash and drugs flowing through pill mills that they had to hire heavily armed guards.
The balance started shifting in 2012 and the following few years. The US and Canadian governments started taking the opioid addiction problem seriously. They closed pill mills, passed new regulations for prescriptions of opioids, and in some cases banned OxyContin altogether. Purdue didn’t fight back, because its patent on OxyContin was running out anyway. The company had developed a new product, OxyNeo, which is more resistant to tampering. Purdue took a high road, appearing to help authorities solve the problem of the diversion of prescription drugs to the street.
The industry as a whole did fight to protect itself. The US Drug Enforcement Agency (DEA) saw what was happening, and started insisting on its power to combat not just street drugs, but the pill mills, the wholesale distribution companies, and even the pharma companies themselves, which were the largest participants in the drug trade. Over the course of a decade, the pharmaceutical industry created legislative momentum for a bill that it wrote, eventually to be known as the ‘Ensuring Patient Access and Effective Drug Enforcement Act’. The Act, which was passed in 2016, established routes by which the DEA could consult and communicate with pharma companies, but prevented the agency from following prescription drugs up the chain. This ensured that the DEA couldn’t investigate distribution or pharma companies. Although the DEA was vehemently opposed to the Act, it was muzzled by a two-part strategy: DEA employees were systematically offered jobs working directly or indirectly for the industry – altogether, fifty employees moved – and well-funded legislators made their more general support of the agency contingent on its staying silent about the Act.74 Most legislators who voted for the Act didn’t understand its implications. The lead Congressman sponsoring the Act, Representative Tom Marino of Pennsylvania, was briefly President Trump’s choice to be ‘drug czar’, until the story of his work against the DEA was revealed.
The situation also changed because of the Sinaloa Cartel in Mexico. The cartel saw its revenue from marijuana plummeting as a result of efforts to legalize the drug. The Cartel, led by Joaquín Guzmán Loera, better known as ‘El Chapo’, needed to change its business model. Since marijuana had become less profitable, it replaced that crop with another: poppies. Leveraging its experience in the marijuana trade, it set out to dominate the US heroin market.75 The result was a 75% drop in the price of heroin, previously sourced from such places as Afghanistan and Pakistan. As the price of Oxy rose and that of heroin fell, users made the switch en masse. Purdue, Abbott and the other companies selling opioids had established their clientele, and the Mexican cartels simply took their customers. Other Mexican cartels soon followed the Sinaloan lead, and also moved into the fentanyl trade.
Although pharma has ceded a large share of the opioid trade to the Mexican drug cartels, some pockets of the industry continue to compete for – and thereby increase the size of – segments of the market. US annual sales of OxyContin peaked in 2011 at nearly $3 billion, but Purdue is vigorously expanding internationally to make up for declining US figures. The company’s owners have an international consortium of companies, Mundipharma, and are working to convince doctors in Southern Europe, Latin America and Asia not to fall into the trap of ‘opiophobia’, an affliction of doctors that leaves patients suffering from chronic pain.76 The campaign is following some standard paths by recruiting KOLs to speak about pain management and the available drugs. Purdue has also pursued the youth market, by testing OxyContin on children – a move that also briefly extended its US patent on the drug. As one commentator writes, ‘OxyContin for kids: What could possibly go wrong?’77
Other pharmaceutical companies are looking for ways to compete in the North American market, too. In late 2016, seven executives and managers who had worked for Insys Therapeutics were arrested in connection with an alleged programme to bribe doctors to prescribe Subsys, a spray that includes fentanyl; in addition, three of the top Subsys prescribers have been convicted of felonies. The supposed bribes were disguised as consulting and speaking fees.78 For their part, some doctors have been happy to profit handsomely. Two Alabama doctors were convicted in 2017 on a raft of charges connected with enormous numbers of prescriptions of Subsys and another fentanyl product, Abstral. The doctors had been accepting kickbacks from Insys for prescribing Subsys for a variety of unapproved conditions. They were also attempting to manipulate the stock price of the relatively small company Galena Biopharma, the maker of Abstral, in the process becoming the top two prescribers of Abstral in the US.79 Meanwhile, in a familiar move, Galena has established a coupon programme to give away the first month’s worth of Abstral.80 What could possibly go wrong?