28
Petri dish (boîte de Petri, Petrischale)
Mathias Grote
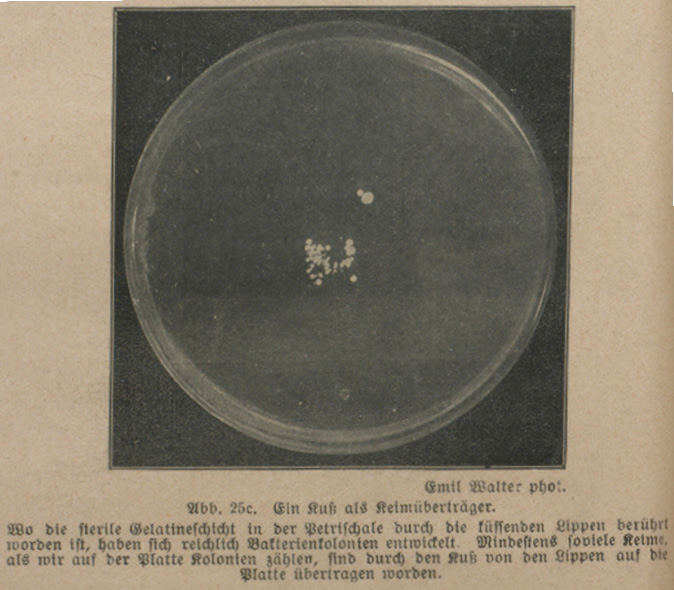
Fig. 28.1 Petri dish (photo by Emil Walter, 1919. Kosmos)
Size and shape: two circular dishes in the shape of a shallow cylinder; c. 10 cm in diameter and 1–1.5 cm in height, differing slightly in size to fit into each other. Colour: transparent, made from either glass or, since the 1950s, thermoplastic materials such as polystyrene. Usage: container or culturing device for various organisms. The smaller cylinder holds solid culture media, liquids, or organisms; the larger one, with its slightly craned rim, functions as a cover. Closed dish creates artificial, sterile environments for the growth or maintenance of diverse organisms (e.g. microbes, plants, cell cultures); also used as a container for chemical reactions with a large surface-to-volume ratio. Origin: devised in 1887 by Richard Julius Petri, medical bacteriologist and member of Robert Koch’s team. Distribution: found in biological and chemical laboratories worldwide, from routine bacteriological diagnostics to reproductive medicine, biotechnologies or research in the molecular life sciences. Status: omnipresent, mundane disposable of the life sciences laboratory that has become proverbial as the site of an experiment, or for the artificial reproduction of organisms.
Keywords: enclosing, isolating, containing, creating a milieu, culturing, storing
The Trace of a Kiss in a Box
‘Wo die sterile Gelatineschicht in der Petrischale durch die küssenden Lippen berührt worden ist, haben sich reichlich Bakterienkolonien entwickelt’ [Ample bacterial colonies have formed where the sterile gelatine layer within the Petri dish has been touched by the kissing lips] (Oettli 1919: 64). This microbial trace of a kiss – in the form of whitish spots, certainly less indelible than its memory – informs an early twentieth-century reader that germs are everywhere. They fall down from the air like snowflakes, as Versuche mit lebenden Bakterien [Trials with Living Bacteria] puts it, this being a small book which appeared in the German popular science series KOSMOS (Figure 28.1).
The Petri dish is an article as mundane yet as indispensable to biological laboratories as a plate is to a kitchen. When catching the trace of a kiss, its function to the authors of Versuche mit lebenden Bakterien was obvious: the dish provided a protected, sterile environment for the growth of a microbial sample. Without such a box, it would have been hard to detect any specific bugs within the plethora of microbes populating every part of our biosphere; the traces left by the lips would have rapidly been contaminated or outgrown. The imprint of the kiss as depicted in this amateur manual rendered the box itself somewhat elusive. Against a dark background, we can only discern the Petri dish’s circular halo from above. This refers to another of the dish’s characteristics: its transparency allows rapid inspection of the organisms with a microscope or even with the naked eye, in this way making possible precise manipulations.
The Petri dish has become an archetypal box of the twentieth-century experimental life sciences. Whether made of glass or plastic, the Petri dish, the test tube, and the Eppendorf cup exemplify the concept of ‘in vitro’ in the life sciences. Organisms, or their bits and pieces (cells, biochemical substances) are enclosed in controlled environments (media, buffers) for culture or examination (Rheinberger 2009; on glassware and ‘laboratory ecology’, see Espahangizi 2015). Petri dishes continue to serve as containers for the growth, display, manipulation, and storage not only of bugs (from bacteria to insects), but also of our bodies’ components, such as cultured cells.
A Box to Purify and Re-produce Microbes
The twin glass dishes fitting loosely into one another were presented somewhat modestly by their eponym, Julius Richard Petri, as ‘Eine kleine Modification des Koch’schen Plattenverfahrens’ [A small modification of Koch’s plate technique] (Petri 1887). No longer than a page, Petri’s contribution was nevertheless well placed in bacteriology’s heroic age, and the device rapidly became adopted. A few years before, Robert Koch, who had just demonstrated anthrax infection, had also introduced solid media for culturing bacteria. Among these were gelatine and agar-agar – reportedly on the advice of a staff member’s wife, who knew the substance as a gelling agent for food preparation (Collard 1976). The liquid media that microbiologists such as Louis Pasteur had used before, e.g. heat-sterilised broth or other types of infusions, constantly mixed the microbes grown therein. This rendered it impossible to determine which cell had developed from which, that is, to obtain ‘colonies’, solid masses of bacteria such as those planted by the kissing lips.
Koch devised solid culture plates by pouring broth mixed with a gelling agent onto a glass surface. This almost ludicrously simple innovation allowed him to make a powerful argument in a controversy raging in late nineteenth-century bacteriology between the so-called monomorphists (Koch himself, and his former mentor, Ferdinand Julius Cohn), who assumed that stable bacterial species existed, and the opposing pleomorphists, who argued that the microbial realm manifested a continuity of life forms that changed in cell shape and physiological effects through life cycles and in reaction to varying environments (Gradmann 2005). Once a microbial sample – isolated, for example, from an infected animal – was growing immobilised on the surface of Koch’s plates, ‘Reinkulturen’ [pure cultures] could be obtained. Such colonies, spots similar to those in our image, were understood as clonal lines, stemming theoretically from a single cell that had divided billions of times. These cell materials could then be transferred to different environments, such as an animal’s body or a different plate, allowing observers to monitor the stability of traits such as cell shape, colour, appearance, causation of disease symptoms, and so on.
Koch accused the pleomorphists of lumping different bacteria together, and advised researchers to follow the principles he saw operative in botany and zoology, namely to distinguish all forms of bacteria differing in traits, as long as their identity or close relatedness had not been demonstrated (Koch 1881). The culture plate, which actually allowed researchers to physically separate and discern microbes by growing pure lines in the laboratory, was a tool for this splitting of microbial diversity. Moreover, the plate and the linked ideal of pure culturing allowed the monomorphists to turn the controversy over the existence of stable bacterial species akin to those of plants and animals into a matter of good versus bad technique. Pleomorphists appeared simply as those using the wrong tools, and towards the end of the nineteenth century, bacteriology rapidly adapted monomorphism. The debate was never really closed however – some decades later, bacterial variability, their interactions with the environment and ideas of ‘life cycles’ rose to prominence again in pathology and beyond (Amsterdamska 1991, Méthot 2015). Ludwik Fleck, in the inter-war time a microbiologist, deliberated about the issue at length, wondering why bacteriology had for a while hardly seen phenomena of variability. In his 1935 monograph, he thus took the ‘rigid thought style’ of the ‘classic Pasteur-Koch era’ as a prime example for his epistemological argument (Fleck 1980: 122; my translation).
The solid culture plate was only one part of the ensemble needed to take a kiss into the laboratory. The other part was the container of this plate. Early users of Koch’s plate method had faced technical problems when pouring the agar onto glass plates, which had to be evenly labelled with the help of a large apparatus. Moreover, the plates needed to be kept free from contamination by airborne microbes, present even in the cleanest and most disciplined bacteriological laboratory. This was achieved by storing them under large bell jars, which one would need to lift in order to manipulate plates or cultures (Collard 1976). Enter Petri: the later director of a tuberculosis sanatorium (described by one of his staff members as a ‘stalwart, priggish Prussian schoolmaster’, Plesch 1951: 40 f.) suggested pouring the inoculated liquid gelatine directly into a small, sterilised glass dish: ‘Geschieht dies, indem man die überfallende Schale nur wenig lüftet und unter ihrem Schutze das […] Gelatineröhrchen ausgiesst, so hat man nur äußerst selten Verunreinigungen durch Luftkeime zu gewärtigen.’ [If this is done by lifting the top dish only slightly and pouring out the gelatine-containing test tube under its cover, one has to face contamination by airborne germs only extremely rarely] (Petri 1887: 279; on Petri, see Plesch 1951: 40 f.). Petri’s dishes rapidly became a staple of the bacteriological laboratory, as they facilitated the adoption of Koch’s plate method. The dishes’ small size made for greater ease of handling in the lab, and they could also be stacked upside down in an incubator, which prevented condensation forming on the plate. The cultures could also be easily inspected through the flat lid (see Collard 1976). A 1915 catalogue of the Berlin instrument-making company F. & M. Lautenschläger, a purveyor to bacteriological and chemical laboratories as well as medical facilities, offered 100 ‘Doppelschale[n] nach Petri, Modell des Instituts für Infektionskrankheiten, Berlin’ [double dishes according to Petri, model of the Institute of Infectious Diseases, Berlin] at a price of 32.50 Marks. This was the suggested number to equip a medium-sized lab (F. & M. Lautenschläger 1915: 486, 687).
Picture the Petri dish in an investigator’s hand: thumb and index slightly lift its cover, the other hand carefully effuses a test tube’s content into the lower dish, before the cover is lowered again to close the dish. This key routine of microbiological lab work has been described and depicted over and over again in various textbooks and manuals during the past century.1 Yet words and images can only do so much. Every student, from medics to biotech, from Bangalore to Cambridge, has to learn the handwork to handle microbes aseptically by imitation in practical courses. It is almost a ritual of microbiology to master holding plugs and test tubes in one hand while fiddling around with a Petri dish in the other, all items close to a Bunsen burner flame. And for good reason: being able to transfer microbes in a sterile manner from one box into another (i.e. by pouring out a test tube into a dish, or by streaking a sample onto a culture plate’s surface) is a prerequisite to properly carry out any microbiological experimentation. If a lab worker does not master these routines under all conditions (such as ill-fitting lab coats, ambient temperature of 35°C, blaring radio, or colleagues calling for lunch), doubt can be cast on any result of culturing, since – as the kiss told us – germs are everywhere. For a technician or a doctoral student, it is not uncommon to carry out these plating routines dozens of times every day. Many of the early breakthroughs in molecular genetics were based on not much more than a few strains of bacteria and phage cultured on plates in Petri dishes. French molecular biologist François Jacob vividly remembered the sound of breaking glass mixed with the warm and sweet smell of agar and rising rage against himself for having impatiently thrown over a pile of dishes that may have harboured a long looked-for mutant colony (Jacob 1987, 315).
If one does not want to kiss the agar in a Petri dish, a number of other simple tools are needed to transfer microbes under sterile conditions. Among these are certainly a Bunsen burner (to rapidly disinfect utensils by heating them), cotton or rubber plugs to seal test tubes, and a glass or platinum wire spatula to transfer the microbial materials. Most of these inevitable little helpers – one may speak of ‘microbiology’s dishes and cutlery’, or of its experimental infrastructure – were devised in the heroic age of Koch and Pasteur. Just like the Petri dish, they are still operative in today’s labs, and they have changed surprisingly little over more than a hundred years. The longue durée use of omnipresent laboratory tools such as these is a peculiar example of continuity in science, which deserves more profound historical scrutiny. An answer to why these tools became so widely used, and why they have remained relatively unchanged, has to address details pertaining to design, practicality, or social factors; moreover, alternative approaches and technologies would need to be studied.2
Even when plastic Petri dishes were introduced in the 1950s, this did not significantly change their simple but essential function. Certainly, the ‘disposable culturing device’ made laboratory life easier; plastic dishes were less prone to slide when wet, or to shatter when falling of a table (with potentially disastrous consequences); hence they were also more easily transported, e.g. by mail, and they could be made to comprise different compartments (Fisk 1959). Moreover, contamination by fungi or viruses through insufficiently cleaned glassware did not occur with a disposable. This latter point, and the different adhesive properties of plastic surfaces, were particularly important for cultures of animal or plant cells, as these often grew attached to the dish, bathed in a liquid medium – which brings us to the Petri dish beyond microbiology.
Between Tumours and Metaphors – the Petri Dish in the Molecular Life Sciences and Beyond
In the twentieth century, cell and developmental biology adopted the Petri dish, among other devices and routines from microbiology, for culturing cells and tissues of higher organisms. Thereby, bodies of animals and humans became amenable to manipulations similar to those described above, and pure mass reproduction of, for example, cancer cells in artificial environments could be achieved. Petri dishes, in combination with other culture plates, flasks, and so on, allowed researchers to expose, study, transfer, and store parts and processes of organisms that had hitherto been hidden in their insides (on cell culture, see Landecker 2007). Moreover, in the second half of the twentieth century, the Petri dish became an important device for screening routines, such as in the ‘Ames test’, which allows the detection of potentially carcinogenic chemicals by incubating them with microbes on a culture plate and looking for mutant colonies (Creager 2014). The early biotech age even saw the construction of a robot to screen large numbers of Petri dishes automatically, and nowadays a number of specially adapted dishes, such as microplates, are on the market (on the screening robot, see Vettel 2006). The classical Petri dish however, its design fitting so well into a human hand, remains a staple of quotidian work. In combination with an arsenal of other tools and skills to put life into boxes, it has therefore become an emblem for artificial reproduction in the laboratory.
At least since the 1980s, the term ‘Petri dish’ has become a trope in literature and the press. One may suspect that this resulted from the 1970s/80s discourse on clones, i.e. identical copies of animals or humans created by scientists. This discourse reflected developments in both genetic engineering and organismic cloning, that is, the transfer of nuclei from somatic cells into enucleated frog egg cells, which resulted in genetically identical animals – a procedure that became widely publicised again in the 1990s with the cloned sheep, Dolly. Cloning in the lab rapidly cross-fertilised with fiction and movies then as now, not only taking up older imaginations (think of the bottled babies in Aldous Huxley’s Brave New World) but also giving the figure of the clone a time-specific guise regarding questions of personal identity or character (Brandt 2009). Importantly, human in vitro fertilisation as practised routinely since the 1980s seems to actually have relied on Petri dishes as the site for bringing isolated egg and sperm cells together (Ebner and Dietrich 2013). While newspapers baptised Louise Brown and her followers according to the various pieces of glassware in which ‘they’ may have floated in their morula stages (‘test tube’, ‘retort’ or ‘Petri dish babies’ have all been mentioned), Petri’s dish became used to refer to more than just living beings (re)-made.3 Even more so than the test tube, the Petri dish turned into a metaphor for ‘a place, environment, etc. in which rapid growth or development can take place’, as the Oxford English Dictionary has it (Oxford English Dictionary 2005). Thus, when The New York Times asserted in a 1984 piece on contemporary dance that ‘[t]he Lower East Side has traditionally been a source, a petri dish of New York culture’, this certainly did not refer to reproducing the identical in an artificial environment behind glass walls, but to a site of sprawling, experimental life (Gautier 1984: 20).
The Teeming Microworld and the Dialectics of Boxing Life
Back to the kisses’ imprint in a Petri dish. This experiment suggested to the amateur microbiologists of 1919 that they were surrounded, and in fact inhabited, by a plethora of whirling, invisible creatures. A sterile Petri dish revealed that not only disease, rot, and decay, but also soil, ‘fresh’ food, and the air they breathed, not to mention their own hands and mouths, were teeming with bacterial life. In fact, the amateur manual seems much more relaxed about microbial presences than one might expect, bearing in mind the ‘microbe hunting’ ideal of early medical bacteriology (Anderson 2004, Sarasin 2007). Certainly, the instructions urge hygiene and care when handling microbes, suggesting for example that experimenters who detect spirochaetes in their mouths should brush their teeth more frequently. However, the manual’s author – the Swiss high school teacher, amateur botanist, and temperance activist Max Oettli – seems to have been positively fascinated by what he called his ‘Versuchspflanzen’ (experimental plants), that is, the ‘Spaltpilze’ (fission fungi, modern bacteria) and the ‘Sproßpilze’ (shoot fungi, modern yeasts; on Oettli, see Spöring 2014).4 Oettli elaborated on the usefulness of microbes in food production – elderflower lemonade and Sauerkraut were on the menu – and he evoked the awe that luminous bacteria obtained from marine fish would create in youngsters and the ‘Naturfreund’ (nature lover) generally.
With his fairly sympathetic attitude to the copiousness and wonder of what teems in and among us, and what small creatures can do, Oettli appears unexpectedly contemporary. Recent scientific and popular discourse abounds with similar tropes about ‘friendly microbes’ – when diseases are not considered infection events per se or when microbial communities are described as global players in biogeochemistry and climate, as helpers of our own metabolism, or as biotechnological all-rounders for the ecological production of biofuels, synthetics, and so forth (O’Malley 2014). And ‘biohackers’ – perhaps the most recent avatar of Oettli’s Naturfreunde – still delight in inoculating their Petri dishes with chunks of mackerel from the fishmonger next door, to marvel at bioluminescent Vibrio fischeri.
The ways in which the modern life sciences enquire into microbial diversity are obviously quite different from those available a century ago. Today, metagenomic technologies allow scientists to probe the diversity and interconnectedness of the microbial world without putting samples and cells into Petri dishes. This is achieved by preparing the DNA of the entire microbial community, for example, in a sample of seawater or from a cow’s rumina. This DNA is then digested into short strands, which are sequenced and assembled in silico to the genomes of organisms assumed in the sample, many of which have never been successfully cultured (O’Malley 2014). By contrast, Oettli and microbiologists in general until the 1990s, first needed to culture the microbes behind glass walls, until colonies became amenable to inspection and manipulations with an investigator’s hands and eyes.
This dichotomy of pure culture versus culture-independent microbiology catches only part of the picture and deserves a more extended analysis. Microbial ecologists such as Sergei Winogradsky, for example, repeatedly criticised pure culturing throughout the twentieth century, and came forward with other methods of studying diversity, such as enrichment cultures – for which other containers were developed (on Winogradsky, see Ackert 2013). Moreover, even our days of high-throughput DNA sequencing cannot abstain from culturing: the question of the organism behind the genome remains pertinent when it comes to classification or (obviously) to using microbes.
Petri’s sterile, transparent, double glass dish, an epitome of pure, controlled life, has nevertheless been arguably the most important tool since the late nineteenth century for making sense of the microcosm by isolating and purifying microbes. Even though the limitations of studying pure cultures in the artificial environment provided by a glass box had been long discussed, it only recently became possible to transcend these limitations on a broad scale. Moreover, while metagenomic DNA sequencing suggests to us nowadays that the microcosm is much more abundant, sprawling, and diverse than assumed in the age of pure culturing, this should not be seen as the last word on the problems of diversity, variability, and specificity within and beyond the lab. Nowadays, a trial person would probably not be asked to kiss a culture plate but instead a cotton bud, which would then be transferred into a plastic Eppendorf cup for DNA preparation. The tools and the specific ramifications of working with microbes may have changed, then, but what has not changed is the need to enclose the diverse microworld in the controlled environment of a box to produce traces (be they cultures or DNA sequences) of what teems in and among us.
Notes
1 Kreuder-Sonnen (2012) analyses how a similar skill (transfer of bacteria between test tubes) was transferred from Koch’s laboratory to Poland in the late nineteenth century – in this case by a meticulous description of the necessary hand movements, illustrated by drawings. In the twentieth century, manuals continued to display series of photographs taken ‘at the bench’.
2 See Grote 2018. On what the ‘longue durée’ could mean for contemporary history of science, see Grote 2015.
3 Numerous such mentions can be found e.g. in The Guardian or The New York Times, with absolute numbers of the term Petri dish rising in the 1980s and 1990s.
4 Today’s fungi and bacteria were considered part of the plant kingdom until at least the mid-twentieth century.
References
Ackert, L., Sergei Vinogradskii and the Cycle of Life: From the Thermodynamics of Life to Ecological Microbiology, 1850–1950 (Amsterdam: Springer, 2013).
Amsterdamska, O., ‘Stabilizing Instability: The Controversy over Cyclogenic Theories of Bacterial Variation during the Interwar Period’, Journal of the History of Biology 24 (1991): 191–222.
Anderson, W., ‘Natural Histories of Infectious Disease: Ecological Vision in Twentieth-Century Biomedical Science’, Osiris 19 (2004): 39–61.
Brandt, C., ‘“In his image” Klonexperimente zwischen Biowissenschaft und Science-fiction’, in Birgit Griesecke et al., eds, Kulturgeschichte des Menschenversuchs im 20. Jahrhundert (Frankfurt: Suhrkamp, 2009), pp. 373–93.
Collard, P., The Development of Microbiology (Cambridge: Cambridge University Press, 1976).
Creager, A. N. H., ‘The Political Life of Mutagens. A History of the Ames Test’, in Soraya Boudia and Natalie Jas, eds, Powerless Science?: Science and Politics in a Toxic World, vol. 2 (New York: Berghahn Books, 2014), pp. 46–64.
Ebner, T., and K. Diedrich, ‘In-vitro-Fertilisation und intrazytoplasmatische Spermieninjektion’, in K Diedrich, M. Ludwig, and G. Griesinger, Reproduktionsmedizin (Berlin: Springer, 2013), pp. 215–24.
Espahangizi, K., ‘From Topos to Oikos: The Standardization of Glass Containers as Epistemic Boundaries in Modern Laboratory Research (1850–1900)’, Science in Context 28 (2015): 397–425.
Fisk, R. T., ‘Disposable culturing device’, US Patent 2,874,091; 17 February 1959.
Fleck, L., Entstehung und Entwicklung einer wissenschaftlichen Tatsache (Frankfurt a. M.: Suhrkamp, 1980 [1935]).
Gautier, R., ‘Reinventing Dance at the Grass Roots’, The New York Times, 3 February 1984, p. 20.
Gradmann, C., Krankheit im Labor. Robert Koch und die medizinische Bakteriologie (Göttingen: Wallstein, 2005).
Grote, M., ‘What Could the ‘longue durée’ Mean for the History of Modern Sciences?’, Working Paper Fondation Maison des Sciences de l’Homme, FMSH-WP-2015–98 (2015), <https://halshs.archives-ouvertes.fr/halshs-01171257>.
Grote M., ‘Petri dish versus Winogradsky column: a longue durée perspective on purity and diversity in microbiology, 1880s–1980s’, History and Philosophy of the Life Sciences 40 (2018), 11.
Jacob, F., La statue intérieure (Paris: Seuil, 1987).
Koch, R., ‘Zur Untersuchung von pathogenen Mikroorganismen’ [1881], in Julius Schwalbe, ed., Gesammelte Werke von Robert Koch, vol. 1 (Leipzig, 1912), pp. 112–63.
Kreuder-Sonnen, K., ‘Wie die Mikroben nach Warschau kamen’, NTM Zeitschrift für Geschichte der Wissenschaften, Technik und Medizin 20 (2012): 157–80.
Landecker, H., Culturing life: How cells Became Technologies (Cambridge, MA: Harvard University Press, 2007).
Lautenschläger, F. & M., ed., Komplette Einrichtung von bakteriologischen, serologischen und chemischen Laboratorien und Untersuchungsanstalten, Operationssälen, Krankenhäusern, Sektionssälen usw. [product catalogue] (Berlin, 1915).
Méthot, P.-O., ‘Bacterial Transformation and the Origins of Epidemics in the Interwar Period: ‘The Epidemiological Significance of Fred Griffith’s “Transforming Experiment”’, Journal of the History of Biology 49 (2016), 311–358.
Oettli, M., Versuche mit lebenden Bakterien. Eine Anleitung zum selbständigen Arbeiten mit Bakterien und anderen Kleinpilzen für den naturwissenschaftlichen Arbeitsunterricht und den Naturfreund (Stuttgart: Franckh’sche Verlagshandlung, 1919).
O’Malley, M., Philosophy of microbiology (Cambridge: Cambridge University Press, 2014)
Oxford English Dictionary, ‘Petri dish’, OED 3rd ed. (2005), <http://www.oed.com/view/Entry/141888?redirectedFrom=Petri+dish&> [accessed 08 Nov 2019].
Petri, J. R., ‘Eine kleine Modification des Kochschen Plattenverfahrens’, Centralblatt für Bacteriologie und Parasitenkunde 1 (1887): 279–80.
Plesch, J., Ein Arzt erzählt sein Leben (München: Paul List Verlag, 1951).
Rheinberger, H.-J., ‘Experimentalsysteme, In-vitro-Kulturen, Modellorganismen’, in Birgit Griesecke et al., eds., Kulturgeschichte des Menschenversuchs im 20. Jahrhundert (Frankfurt a. M.: Suhrkamp, 2009), pp. 394–404.
Sarasin, P., et al., ed., Bakteriologie und Moderne: Studien zur Biopolitik des Unsichtbaren 1870–1920 (Frankfurt a. M.: Suhrkamp, 2007).
Spöring, F., ‘“Du mußt Apostel der Wahrheit warden”: Auguste Forel und der sozialhygienische Antialkohol-Diskurs, 1886 – 1931’, in Judith Große, Francesco Spöring and Tschurenev, J., Biopolitik und Sittlichkeitsreform: Kampagnen gegen Alkohol, Drogen und Prostitution 1880–1950 (Frankfurt: Campus Verlag, 2014), pp. 111–44.
Vettel, E. J., Biotech: The Countercultural Origins of an Industry (Philadelphia: University of Pennsylvania Press, 2006).