31
The Mirror Trap
Etienne S. Benson
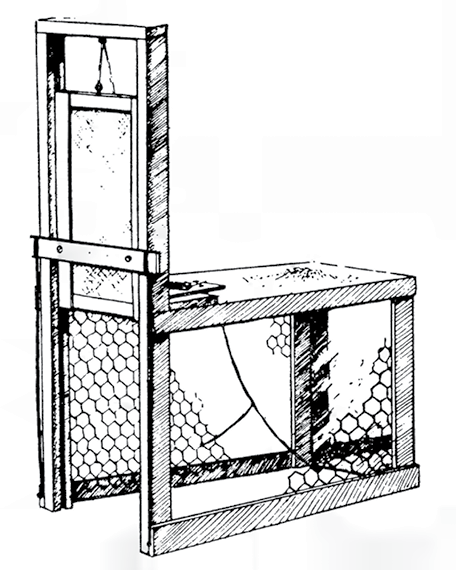
Fig. 31.1 ‘Mirror trap set, showing trigger mechanism, mirror, and rubber bands, fastened to bottom of door’ (source: Figure 1a in J. F. S. Bendell and C. David Fowle 1950: 481)
Box: the mirror trap. Size: 28 inches long, 14 inches wide, 18 inches high. First observed: Pennsylvania, 1947. Distribution: North American forests, often found on or near the drumming logs of ruffed grouse. Behaviour: sudden, automatic, sometimes violent. Sexual dimorphism: none (asexual: a box), but made for males. Common uses: trapping birds, exercising coercive care, affirming human exceptionalism. Possible risks: serious injury, profound misrecognition. Status: rare but not endangered. Sometimes mistaken for: a rival, oneself.
Keywords: captivating, caring, confining, injuring, preserving, managing, recognising, misrecognising
The mirror trap was invented in the spring of 1947 by Glenn L. Bowers and Ward D. Tanner, two graduate assistants working for the Pennsylvania Cooperative Wildlife Research Unit (Bowers and Tanner 1947, 1948). Its design is simple but ingenious.
The trap itself is fairly ordinary: a box of wood and wire mesh measuring 28 inches long, 14 inches wide, and 18 inches high, with a spring-loaded sliding door at one end that is triggered by a treadle on the floor of the trap. It is the bait that is unusual. One of the inner sides of the box is fitted with an 8-by-10-inch mirror. That’s it. No alluring scents, no tempting morsels, no beguiling camouflage.
For at least one particular target, however, the mirror is enough. That target is the ruffed grouse, a species distantly related to the chicken that can be found in early successional forests across North America (Dessecker and McAuley 2001). The ingenuity of the trap lies in its simplicity, which in turn depends on a single penetrating insight into the grouse’s capabilities and vulnerabilities.
In the mating season a male ruffed grouse will attack other males who venture too close to the fallen log, rock, or other site he has selected for his ‘drumming’ display. (The sound is actually made by the rapid beating of the bird’s wings). Unable to recognise the image reflected in the mirror as his own, the grouse rushes toward his imagined competitor, triggering the trap’s release mechanism and sealing himself inside the box. In the grandiloquent terms of the press release issued by the Unit once the success of the trap had become clear in the spring of 1947: ‘The King Succumbs to his Own Image’ (Pennsylvania Cooperative Wildlife Research Unit 1947a). At a time when grouse were scarce in the state of Pennsylvania, the new design seems to have dramatically facilitated their capture (Bowers 1947).
I have been unable to find any detailed account of the trap’s genesis. Did Bowers and Tanner know of the ancient Roman belief that a tigress pursuing her stolen cubs could be distracted by a mirror, which she would misrecognise as the very offspring she was trying to rescue (Toynbee 1996: 72–81)? Were they familiar with the miroir aux alouettes, a spinning block of wood embedded with small mirrors that was used to snare larks and other songbirds in early modern Europe? (The device seems to have attracted birds by its shiny, water-like surfaces rather than by reflections of their own images (see Arentsen and Fenech 2004)). Were they simply struck by inspiration after spending long hours in the field watching the antics of amorous birds from afar? However it came about, the box they built embodied a refined understanding of the grouse’s world.
All successful traps require some such understanding. The target must walk, fly, swim, or slither into the trap of his or her own volition; this is what distinguishes a trap from a weapon. And so the trapper must know something of the target’s habits, desires, and capabilities, so that they can be used against him. Like a hunter, he must imagine himself into the animal’s life, perhaps even see himself as animal (Willerslev 2007); unlike the hunter, he must embody this knowledge in an apparatus that will operate in his absence (Kassung 2012). A collection of all the traps ever made would be a treasure-trove of such knowledge in material form, a kind of map of animal worldviews in reverse. It would be a very incomplete map, since the hardest part of trapping is often knowing when, where, and how to place the trap, but it would be a wonder nonetheless.
Much of this fantastical collection would consist of devices that kill or maim: the leghold traps, the deadfalls, the snares, the spiked pits, and the drowning sets (see Spencer 2007), as well as those miniature modern horrors, the glue boards, ‘responsible for more suffering than virtually any other wildlife control product on the market’ (Humane Society of the United States 2012). But a good portion would consist of live traps, of which the mirror trap is a specialised variety.
The live trap requires an even more intimate knowledge of the animal than the death trap. Not only must it be designed in such a way as to take advantage of the animal’s blind spots, but it must also allow the animal to survive within the trap until the trapper arrives, which may take hours or even days. It thus combines a ruthless exploitation of the animal’s vulnerabilities with a limited but genuine concern for his survival. The slamming shut of the trip-wired door is the moment that divides the two.
Even when an intent to injure is absent, there is a violence inherent to such traps. One of the first grouse caught in Tanner and Bowers’ trap died before the trap was inspected, probably as a result of wounds sustained in his frantic attempt to escape (Bowers 1948: 4). Another ‘succumbed’ after being removed from the trap and photographed (Pennsylvania Cooperative Wildlife Research Unit 1947b: 14). Many others were severely injured within the trap, despite a lining of soft netting having been installed on the inside of the top panel to cushion their collisions. Scalping was particularly common, if rarely fatal. Later refinements further reduced but never eliminated the possibility of injury (Bendell and Fowle 1950; Dorney and Mattison 1956; Chambers and English 1958; Gullion 1965). Getting the bird into the box was difficult; keeping him alive and well once inside was even harder.
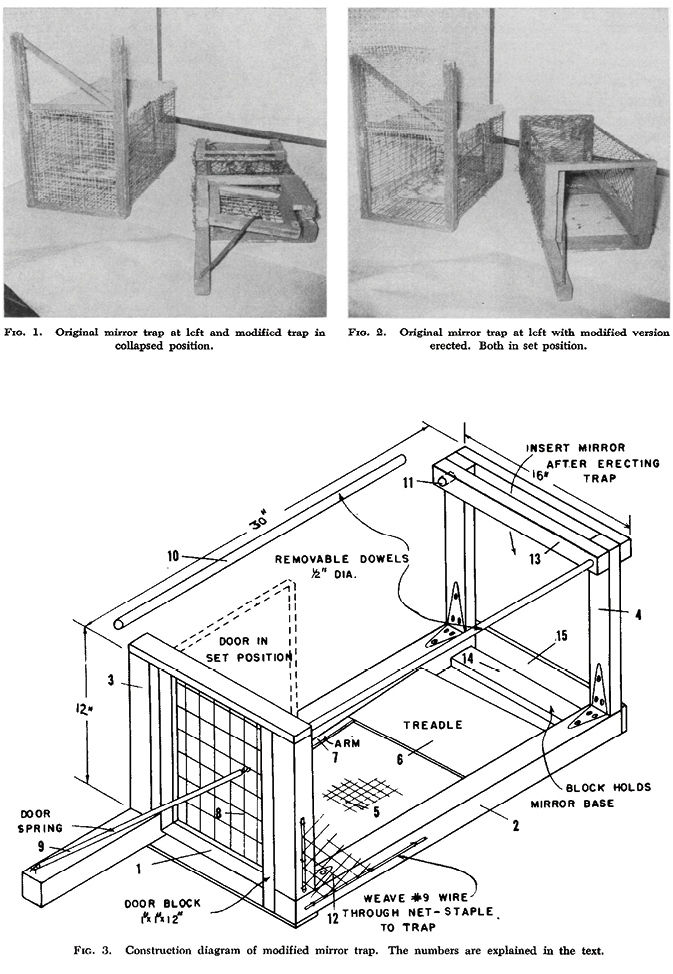
Fig. 31.2 A modified design of the mirror trap that could be collapsed for easy transportation in the field (source: figures 1–3 in Chambers and English (1958, 200–2))
There are many reasons for wanting to trap an animal alive. One is to ensure, before killing him, that he is actually the kind of animal one wants to kill. Another is to bring him into captivity – that is, to move him from the box in which he was caught to the cage, paddock, tank, or enclosure in which he will live out the rest of his life, short or long as it may be. Neither of these was Tanner and Bowers’ goal. Instead they used the trap to hold the grouse in place, just for a moment, so that they could weigh, sample, band, mark, photograph, and release him. Grouse biologists today continue to use versions of the mirror trap for similar purposes (e.g., Zimmerman and Gutiérrez 2007).
The figure of the live trap encapsulates some of the basic axioms of twentieth-century wildlife management, a field that emerged in the wake of failures to preserve wild animals simply by placing prohibitions on killing them (Dunlap 1988; Reiger 2001; Newton 2006). Setting limits on the animal’s freedom, the trap materialises a belief in a limited biological world, hemmed in by human activity, which only the reasoned application of science and technology can redeem. In this context, rather than being a tool of exploitation, the trap becomes a means of conservation, however coercive. Paradoxically, it comes to stand for freedom, for it is only because of the knowledge produced through trapping that grouse can continue to pursue their lives and interests in a landscape dominated by humans.
Or rather, one should perhaps say, in a landscape dominated by ‘man’, since the gendered aspects of live-trapping are difficult to ignore. For most of the history of live-trapping of oft-hunted ‘game’ birds such as grouse, the field has been dominated by male biologists, hunters, and conservationists. Care is often associated with the feminine, but perhaps this is only because attention to care has tended to focus on its most maternal and nurturing aspects (e.g., Gilligan 1982; see Gardiner 2002). When a male wildlife biologist seeks to manage a ‘king’ among birds — the very same king whom he and his fellow hunters may later seek to kill — care wears another face. Such patriarchal care can be empathetic but coercive, other-oriented but ego-affirming; it can foster individual flourishing even as it reinforces power, custom, and law.
Such forms of coercive care are not pretty – perhaps not even good, in some absolute sense – but they are not necessarily any less genuine for that. For the grouse, which is counted among the most valued game birds of North America, patriarchal care in the twentieth century was linked to a vision of authentic contact with the land through hunting that could only be preserved in the modern world through the use of science and technology (Reiger 2001; see Holsman 2000).
This vision of coercive care and conservation-for-and-through-killing is mirrored, in miniature, in the positioning of biologist and grouse in relation to the mirror trap. To understand the grouse as essentially unable to recognise himself in the mirror is to draw a line between those beings – humans, ‘man’ – who are self-consciously capable of taking responsibility for earthly life, and those who are unconscious victims of forces beyond their comprehension. Each trapped grouse is then living proof of the line’s reality. The grouse’s very inability to recognise himself establishes the ethical ground for human intervention – just as, more broadly, a vision of nonhuman animals as unconscious machines (Stam and Kalmanovitch 1998; Crist 1999) helps undergird some of the more hubristic visions of the Anthropocene, the geological age when ‘man’s’ impact on the Earth rivals the great forces of ‘Nature’ (Crutzen 2002; Crist 2013; Hamilton 2013). In such visions, coercive care appears as an ethical obligation for those who stand on one side of an ontological divide toward those who stand on the other.
At first glance, the mirror trap, consciously built to exploit the grouse’s tendency to mistake himself, might seem like material corroboration of the validity of this view. But divisions like these, so clear from a distance, often dissolve on close inspection.
First it must be noted that the ideal grouse as depicted in the early media coverage of the mirror trap, a ‘passionate male’ who ‘leaps jealously across the trigger-door’ to attack his supposed rival, is just that, an ideal (Associated Press 1947). In practice the mirror trap is most effective at catching very aggressive males and those in areas of high competition, as the great grouse biologist Gordon W. Gullion noted. Timid or wary males may ignore the mirror completely; some become frightened rather than provoked. These more wary birds – one is tempted to call them more intelligent – can sometimes be captured once with the mirror trap, but rarely twice. Such birds, Gullion observed, ‘tend to survive longer than other males’ (Gullion 1965: 112).
The mirror trap is thus doubly selective, trapping not only males rather than females but also the most aggressive rather than the least aggressive males. To see the trap as reflecting something essential about male grouse, or about grouse-ish masculinity, is to make a mistake even more fundamental than the grouse’s misrecognition of himself in the mirror. The mirror trap becomes a norm in the shape of a box, trapping only males who meet certain mid-twentieth-century expectations about what a male should be. Males who fail to meet those expectations – those who think twice before attacking – slip out of view (Despret 2004; Law and Lien 2012).
If all traps embody an intimate knowledge of an animal’s habits, capabilities, and vulnerabilities, all traps embody such misrecognitions, too. The ruffed grouse in the abstract cannot be trapped; only a particular grouse can be trapped, the precise nature of whose divergence from the ideal cannot be known in advance. Traps are thus predictions, even self-fulfilling prophecies, and the knowledge they embody produces its subject as much as it reflects it.
Beyond the specific selectivities of the mirror trap, the automatism inherent to all traps is a prophecy of this sort, pre-emptively framing the human-animal encounter in such a way that the trapped animal must necessarily be the kind of being who can be taken unawares by a mechanism. A kind of ‘latent Umwelt’ becomes manifest, one in which the relation between human and animal is mechanised and objectified (Kassung 2012: 207). In this way a line is drawn between those who are free to respond and those who are doomed to react (Derrida 2002). It is only the latter who end up in the trap, available to be made into objects of science. To ask whether or not grouse really are unconscious animal-machines is to miss the point; the trap itself is a technological means of bringing forth just this reality and not another (see Cussins 1996; Mol 2002; Barad 2007).
At first glance, then, the mirror trap might seem to offer material proof of the existence of a dividing line between self-conscious humanity and the vast unconscious immanence of nature, but on closer inspection the line begins to fracture and blur. Some male grouse, confronted by a mirror image of their bodies, leap to the attack and are trapped. Others, more timid, wary, perceptive, or perhaps even intelligent, keep their distance or flee. Still others proceed unperturbed with their drumming and strutting, for all the world as if a large box with a mirror inside it had not just been placed near the centre of their universe. A small proportion of male grouse seem to never drum at all (Gullion 1981). In one of Gordon Gullion’s early studies with the mirror trap, even the reactions of those who were successfully trapped could only be described as ‘highly variable’ (Gullion 1965: 114). For researchers attentive to the limits of traps and the variability of life, it is clear that no grand generalisations can be made.
If the mirror trap is based on a fundamental misrecognition of the grouse, it is also based on a misrecognition of recognition itself. In the second half of the twentieth century some animal-behaviour scientists began to argue that the ability to recognise oneself in a mirror could serve as an experimental test of self-awareness, even of the existence of a self. That chimpanzees passed this test, it was claimed, provided evidence of ‘self-concept in a subhuman form’; that macaques flunked it provided evidence of a clear line dividing humans and great apes from the rest of the animal world (Gallup 1970: 87). Although this line has since been made more sinuous by the discovery of mirror self-recognition in dolphins (Reiss and Marino 2001) and elephants (Plotnik et al. 2006), it is still used to reinforce the border between humanity and the other animals.
Whatever the validity of such experiments may be, there is nothing simple or self-evident about the idea that one can see oneself in the image reflected in a mirror. The problem is not that the outer surface always belies the more authentic inner depths, but that that inner self is itself fragmented, socially distributed, and non-identical from moment to moment (Lacan 2006). To recognise oneself in a mirror is undoubtedly to prove that one has a concept of self, but it is also to admit that one has been seduced – despite abundant experiential evidence to the contrary – by an illusion of wholeness, autonomy, and self-similarity. It is to be lured by the eye into a trap of one’s own making.
The experimental situations in which mirror self-recognition is tested are just such traps, into which certain animals called scientists invite other animals to fall. Those who do fall are counted as conscious kin. Those who do not are consigned to unconscious animality, to that part of the living world that is, in the words of Georges Bataille, ‘in the world like water in water’ (Bataille 1989: 19; see Tyler 2005). Both grouse and grouse researchers misrecognise themselves, then, if in different ways. Which is the more fundamental error: to mistakenly think one is confronted by another, or to mistakenly think one is confronted by oneself?
Thus, the image of the grouse that is reflected in the mirror trap becomes both partial and multiple, as do the images of all those animals whose vulnerabilities, capabilities, and habits have been exploited by myriad ingeniously designed traps over the millennia. We construct a world of traps that depend on others being unable to recognise themselves just as we do, and then we take their failure to do so as a sign of our superiority and our right, and perhaps even our responsibility, to keep constructing. Our treasure-trove of animal worlds in reverse is revealed to be an embodied collection of misrecognitions – effective, certainly, but hardly the veridical map of otherness it first seemed to be. Certain trappable individuals become objects of knowledge-making, while others live out their lives ‘on the margins of what is knowable to the human’ (Law and Lien 2012: 373). The box is filled with those who fit in it, while the rest remain outside.
References
Arentsen, H. F., and N. Fenech, Lark Mirrors: Folk Art from the Past (Malta: [s.n.], 2004).
Associated Press, ‘Simple Mirror Trap Developed to Catch Wild Grouse Alive’, The Southeast Missourian, 18 December 1947.
Barad, K. M., Meeting the Universe Halfway: Quantum Physics and the Entanglement of Matter and Meaning (Durham: Duke University Press, 2007).
Bataille, G., Theory of Religion, trans. by Robert Hurley (New York: Zone, 1989).
Bendell, J. F. S., and C. D. Fowle, ‘Some Methods for Trapping and Marking Ruffed Grouse’, Journal of Wildlife Management, 14 (1950): 480–82.
Bowers, G. L., ‘Progress Report on a Population Study of the Ruffed Grouse (Bonasa u. umbellus) in Pennsylvania at the Low of the Cycle’, Quarterly Report of the Pennsylvania Cooperative Wildlife Research Unit, 10, Apr-May-June (1947), separately paginated, 9pp.
——, ‘Roughed Grouse Study’, Quarterly Report of the Pennsylvania Cooperative Wildlife Research Unit, 11, Apr-May-June (1948): 2–6.
Chambers, R. E., and P. F. English, ‘Modifications of Ruffed Grouse Traps’, Journal of Wildlife Management, 22 (1958): 200–02.
Crist, E., Images of Animals: Anthropomorphism and Animal Mind (Philadelphia: Temple University Press, 1999).
——, ‘On the Poverty of Our Nomenclature’, Environmental Humanities, 3 (2013): 129–47.
Crutzen, P., ‘Geology of Mankind’, Nature 415 (3 January 2002): 23.
Cussins, C., ‘Ontological Choreography: Agency Through Objectification in Infertility Clinics’, Social Studies of Science, 26 (1996): 575–610.
Derrida, J., ‘The Animal That Therefore I Am (More to Follow)’, trans. by David Wills, Critical Inquiry, 28 (2002): 369–418.
Despret, V., ‘The Body We Care For: Figures of Anthropo-Zoo-Genesis’, Body and Society, 10 (2004): 111–34.
Dessecker, D. R., and D. G. McAuley, ‘Importance of Early Successional Habitat to Ruffed Grouse and American Woodcock’, Wildlife Society Bulletin, 29 (2001): 456–65.
Dorney, R. S., and H. M. Mattison, ‘Trapping Techniques for Ruffed Grouse’, Journal of Wildlife Management, 20 (1956): 47–50.
Dunlap, T. R., Saving America’s Wildlife (Princeton, NJ: Princeton University Press, 1988).
Gallup, G. G., Jr., ‘Chimpanzees: Self-Recognition’, Science 167. 3914 (2 January 1970): 86–7.
Gardiner, J. K., ed., Masculinity Studies and Feminist Theory: New Directions (New York: Columbia University Press, 2002).
Gilligan, C., In a Different Voice: Psychological Theory and Women’s Development (Cambridge, MA: Harvard University Press, 1982).
Gullion, G. W., ‘Improvements in Methods for Trapping and Marking Ruffed Grouse’, Journal of Wildlife Management, 29 (1965): 109–16.
——, ‘Non-Drumming Males in a Ruffed Grouse Population’, Wilson Bulletin 93 (1981): 372–82.
Hamilton, C., Earthmasters: The Dawn of the Age of Climate Engineering (New Haven: Yale University Press, 2013).
Holsman, R. H., ‘Goodwill Hunting? Exploring the Role of Hunters as Ecosystem Stewards’, Wildlife Society Bulletin, 28 (2000): 808–16.
Humane Society of the United States, ‘Glue Boards: Cheap, Cruel, and Indiscriminate’, 29 March 2012, <http://www.humanesociety.org/animals/resources/facts/glue_boards.html> [accessed 13 June 2015].
Kassung, C., ‘Animal Machines. Eine Falle ist kein Ge-Stell’, in T. Conradi, G. Ecker, N. O. Eke and F. Muhle, eds, Schemata und Praktiken (Munich: Wilhelm Fink, 2012), pp. 191–211.
Lacan, J., ‘The Mirror Stage as Formative of the I Function as Revealed in Psychoanalytic Experience’, in Écrits, trans. by Bruce Fink (W.W. Norton & Co., 2006), pp. 75–81.
Law, J., and M. E. Lien, ‘Slippery: Field Notes in Empirical Ontology’, Social Studies of Science, 43 (2012): 363–78.
Mol, A., The Body Multiple: Ontology in Medical Practice (Durham, NC: Duke University Press, 2002).
Newton, J. L., Aldo Leopold’s Odyssey (Washington: Island Press/Shearwater Books, 2006).
Pennsylvania Cooperative Wildlife Research Unit, ‘The King Succumbs to his Own Image’, Quarterly Report of the Pennsylvania Cooperative Wildlife Research Unit, 10. July-Aug-Sept (1947a): 1–2.
Pennsylvania Cooperative Wildlife Research Unit, ‘Ruffed Grouse Study’, Quarterly Report of the Pennsylvania Cooperative Wildlife Research Unit, 10. Oct-Nov-Dec (1947b): 13–14.
Plotnik, J. M., F. B. M. de Waal, and D. Reiss, ‘Self-Recognition in an Asian Elephant’, Proceedings of the National Academy of Sciences of the United States of America, 103 (2006): 17053–57.
Reiger, J. F., American Sportsmen and the Origins of Conservation, 3rd edn (Corvallis: Oregon State University Press, 2001).
Reiss, D., and L. Marino, ‘Mirror Self-Recognition in the Bottlenose Dolphin: A Case of Cognitive Convergence’, Proceedings of the National Academy of Sciences of the United States of America, 98 (2001): 5937–42.
Spencer, J., Guide to Trapping (Mechanicsburg, PA: Stackpole Books, 2007).
Stam, H. J., and T. Kalmanovitch, ‘E. L. Thorndike and the Origins of Animal Psychology: On the Nature of the Animal in Psychology’, American Psychologist, 53 (1998): 1135–44.
Tanner, W. D., and G. L. Bowers, ‘A Method for Trapping Male Ruffed Grouse’, Quarterly Report of the Pennsylvania Cooperative Wildlife Research Unit, 10. Oct-Nov-Dec (1947): 15–16.
——, ‘A Method for Trapping Male Ruffed Grouse’, Journal of Wildlife Management, 12 (1948): 330–31.
Toynbee, J. M.C., Animals in Roman Life and Art (Baltimore: Johns Hopkins University Press, 1996 [1973]).
Tyler, T. ‘Like Water in Water’, Journal for Cultural Research, 9 (2005): 265–79.
Willerslev, R., Soul Hunters: Hunting, Animism, and Personhood among the Siberian Yukaghirs (Berkeley: University of California Press, 2007).
Zimmerman, G. S., and R. J. Gutiérrez, ‘The Influence of Ecological Factors on Detecting Drumming Ruffed Grouse’, Journal of Wildlife Management, 71 (2007): 1765–72.